Beetles offer people lessons in moisture control
Insects inspire new surfaces that are better at collecting water or resisting ice buildup

Onymacris laeviceps is a beetle in the Namib Desert that collects water from fog. This tactic has now inspired human engineers.
james.harris.anderson/Flicker (CC-BY-NC-SA 2.0)
By Sid Perkins
Whether it’s a beetle or a cactus, organisms that live in the desert often have a tough time finding water. In some places, water can be scarce all year long. In others, it’s available only in certain seasons. To cope with these dry conditions, desert-dwelling animals and plants have evolved some interesting ways to slake their thirst. Some get all they need from the animals, plants or seeds that they eat. That means these diners never need to take a drink. Others species have neat tricks for collecting water out of thin air.
Now, engineers are looking to borrow those tricks. In some cases, the researchers want to snatch water from humid air, just as the animals do. They might want to collect the water for drinking or other purposes. Or, they might want to dehumidify the air in areas with poor air circulation. That could stop or slow the growth of mold.
No matter the purpose, these scientists and engineers (researchers who use science to solve problems) have begun borrowing Mother Nature’s designs.
Inspiring insects
One of the world’s loneliest deserts lies along the southwestern coast of Africa. The Namib Desert is about the size of South Carolina. Some spots there average as little as 10 millimeters (0.4 inch) of rain each year. Many sites along the coast get only half that amount. It’s little wonder that few people live there.

In some deserts, the most common sources of water are small pools or pockets of wet sand that can be found underground. But that’s not the case in the Namib, says Andrew Parker. He’s a biologist at the Natural History Museum in London, England. “There’s not much rainfall, and it’s very hot there,” he says. “The only reliable source of water is fog from the sea.”
Some beetles thrive in the Namib Desert because they can tap into that fog for a drink. Scientists have found four species that use this trick, says Parker. These unusual insects differ in size. Some have bumpy shells. Others have grooved shells. But to get a drink, all act the same way. When the fog rolls in, the beetle stands atop the dunes on tiptoe. It then sticks its rear end up into the humid morning air. Fog droplets strike and then cling to the beetle’s back. Or humidity can condense into water right on the insect’s shell. When the drops there grow large enough, they roll off the beetle’s back and down to its mouth for a refreshing drink.
“These beetles are very well adapted to the desert’s harsh conditions,” says Parker. He described the behavior of these insects two years ago in Bioinspiration & Biomimetics. (Biomimetics is the creation of new tools or techniques based on living organisms. It also is known as biomimicry.)
There are many desert creatures that collect water in unusual ways. But the Namib Desert beetles are probably the most studied, Parker notes. Their simple techniques also may be the easiest for engineers to copy, he adds.
But, he admits, “It’s one thing to understand what’s going on in nature. It’s another to actually take advantage of it.”
Try, try again
As any engineer can tell you, the first design for something doesn’t always work. Sometimes the design needs a tweak or two — or dozens. That’s true even when an engineer starts with one of nature’s tried-and-true blueprints.
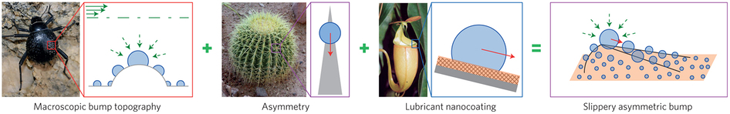
Just ask Joanna Aizenberg. She’s a materials scientist at Harvard University in Cambridge, Mass. She and her team recently built a surface to collect water from the air. They started with a design inspired by the small, dome-shaped bumps on the shells of the Namib’s fog-collecting beetles.
The researchers found that in very humid air, water droplets condense on the peak of each bump. Their tests showed that the smaller the bump, the faster the droplets grow. But then the scientists noticed a problem. The beads of water would grow quickly at first, and then much more slowly. That’s because each growing droplet eventually covered up the peak, where condensation was most efficient.
So the researchers decided to change the shape of the bumps on their test surface. Instead of curved domes, they made these bumps flat-topped boxes with slightly rounded edges. That design left a large flat space on top of each bump for droplets to collect. More importantly, the design kept the growing droplets away from the bumps’ rounded edges, where new water droplets would form most readily.
That new design let the water droplets grow for a longer time. That boosted water collection in the short run, says team member Kyoo-Chul Park. He’s a mechanical engineer at Harvard University. But, as before, the droplets eventually grew to cover the bump. That’s when the team realized something: To keep collecting water, they needed to remove the droplets from those bumps.
So they borrowed a trick from another desert organism — the cactus. Many types of cactus have spines. A spine is typically pointy at its tip and thicker at its base. Having spines helps protect a cactus against plant-eating creatures, of course. But these spines have another benefit as well. Any dew that collects on a spine will be drawn from its pointy end down toward the broader base by capillary action. Water molecules pull each other along the surface they’re resting on. As a result, the water runs toward the plant and then downward to the ground. It’s a design that helps the cactus water itself!
The researchers changed the shape of the bumps on their surface yet again. They made them look like tiny skateboard ramps with narrow peaks and broad bases. With that shape, droplets formed on the bumps, then flowed away. The change, notes Park, kept the bumps mostly free of large droplets. That, in turn, let droplets grow steadily and boosted overall water collection.
Carnivorous plants to the rescue

To help the droplets flow off the bumps more quickly, the researchers borrowed yet another trick from nature. This time, they took a hint from a carnivorous plant called a pitcher plant. These tropical plants get many of their nutrients by consuming animals, usually insects. Pitcher plants have a bucket-shaped structure that often has a very slick rim. Insects attracted to the plant walk on that rim. Many also slip and fall inside to their doom.
So the team changed the bumps once again. This time, they gave the bumps a nubby texture that would help hold an oily coating. That coating made the bumps super slick. They were so slippery that capillary action could pull the water droplets upward, against gravity, if the wide part of the bump were higher than the tip.
The team described its results in the March 3 Nature. In tests done in humid air, surfaces with the slick, oddly-shaped bumps started producing their first water droplets in about 16 minutes. That’s about one-fourth the time needed for other types of surfaces, says Aizenberg. Tests lasting a few hours suggest that their surface actually collects water at six times the speed of other surfaces.
The ultimate goal is to develop big surfaces that can collect drinking water from the air for people who live in desert communities.
These surfaces could be useful many places besides the desert, Park says. For example, they could work in devices called dehumidifiers. These machines use condensation to pull water vapor from humid air inside a home. People use dehumidifiers to breathe more easily or feel more comfortable. They also use them to prevent mold in damp areas such as basements.
Frost-stopping tricks
In the blazing heat of the Namib Desert, the water droplets on a beetle’s bumpy back certainly aren’t at risk of freezing. But their water-collecting tricks just might help engineers design surfaces that can stay free of ice and frost in colder places.
When droplets form on a smooth surface, they appear at random, all over the place. They start out small. But as they grow, they crowd against each other, then join to make bigger droplets. When they get big enough, gravity makes them run off. That leaves room for new droplets to form. (This is what happens on a cold mirror in a humid bathroom, for example.)

But imagine the same process happening outdoors, in the freezing cold. Sometimes, droplets condense on a surface but stay liquid even though air temperatures are below freezing. To become ice, these supercooled droplets need a particle to crystallize around. Now, let’s say a bit of dust floats down into one droplet and triggers a freeze. Because ice is less dense than water, the freezing droplet expands. If the edge of that fresh bit of ice bumps into a neighboring droplet, it can trigger that droplet to freeze — and so on. In the right conditions, that chain reaction can cover a window or windshield in frost.
But maybe there’s a way to stop an ice-up from spreading, says Jonathan Boreyko. He’s a materials scientist at Virginia Tech in Blacksburg. One way to stop or slow the icing process, he suggests, might be to keep droplets from crowding close enough that they touch as they start to freeze.
That’s why the Namib Desert beetles came to mind, says Boreyko. As Aizenberg and her team also noted, droplets condense on a bump more easily than they do in the spaces between them. So isolating droplets on bumps could keep them away from each other. But rather than make bumps on their surface, Boreyko and his team decided to take a slightly different approach. They chose to create water-collecting islands on a surface that repelled water everywhere else.
For their tests, they started with a wafer of silicon. That’s the same sort of material used to make computer chips. They washed the surface with acid to make sure it was clean and free of water-repelling substances. Then they covered the entire surface with a layer of water-repelling polymer. (Think of this like a coat of waterproof paint.) Finally, they used a hot stream of plasma, or atoms separated into positively and negatively charged particles, to etch patterns in the polymer. That step exposed small areas of the silicon below. And because the plasma included oxygen, the etching also created water-attracting patches of silicon oxide.
Fragile surfaces offer proof of principle
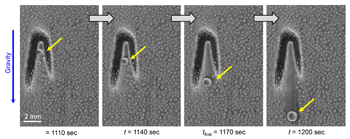
On their postage-stamp-sized samples, the researchers created water-attracting patterns of many shapes and sizes. Some samples were covered with little dots. Others had small squares, triangles or stripes. Sometimes the water-attracting areas were close to each other. For other samples, they were spread farther apart.
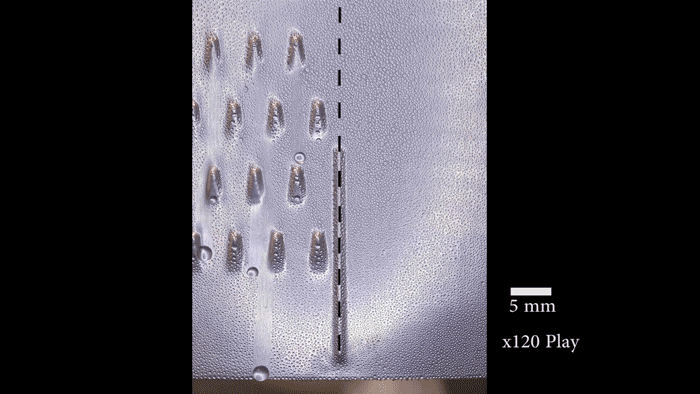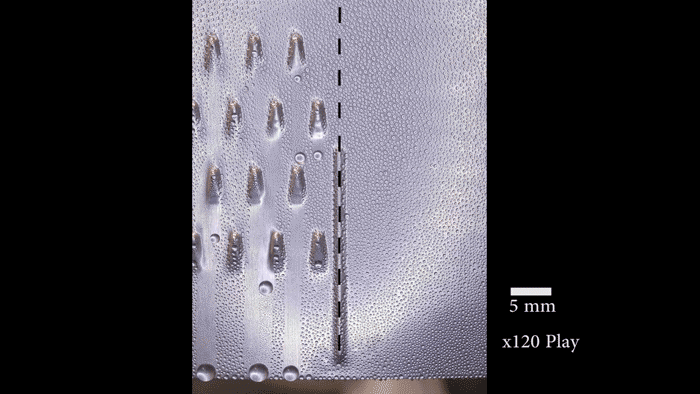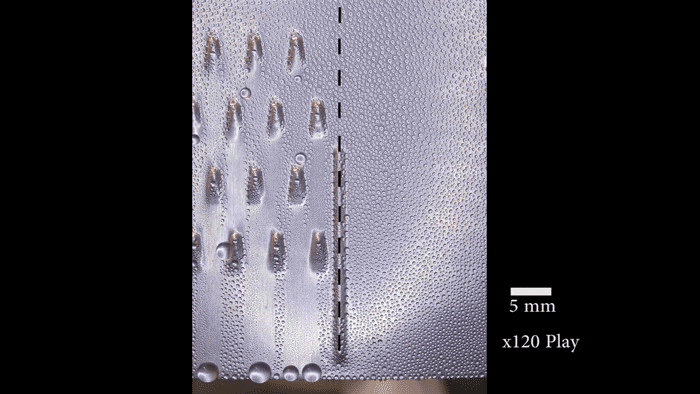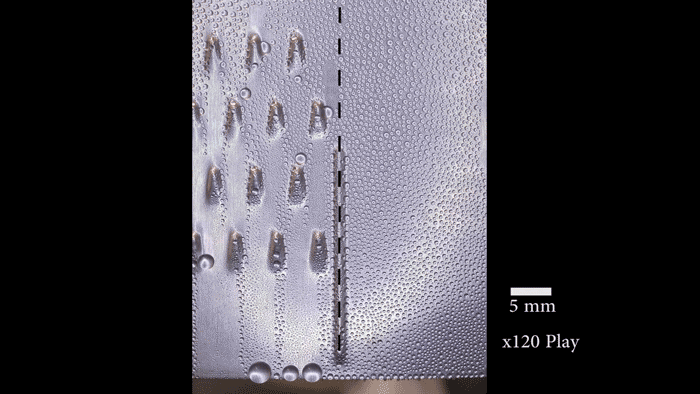
“When the dots are spaced far enough apart, the ice is no longer able to spread frost to the neighboring drops because they’re too far away,” says Boreyko. He and his team described their test results January 22 in Scientific Reports.
The surface areas that Boreyko and his team tested were small patches that measured only 0.5 millimeter (0.02 inch) across. But it should be easy to make the process work on large areas too, he says. Also, the water-repelling layer the researchers added to the surface is delicate. It probably wouldn’t stand up to real-world conditions. However, it should be possible to design sturdier layers that could survive wind, rain and other harsh conditions.
This technology could become widely used, the researchers say. It might help prevent ice from building up on home heating and cooling equipment, for example. It even might help keep ice from forming on airplane wings and wind turbine blades. That could save lots of money because airports wouldn’t have to buy large volumes of deicing chemicals, says Boreyko. Avoiding those harsh chemicals also would be good for the environment.
“By paying more attention to controlling condensation and freezing, we could end up with huge cost savings,” he says.
The new surfaces could help save money and the environment — all thanks to some little beetles with a nifty knack for collecting water.