2019 Nobel Prize in chemistry goes for pioneering lithium-ion batteries
The three winners helped create lightweight, rechargeable devices
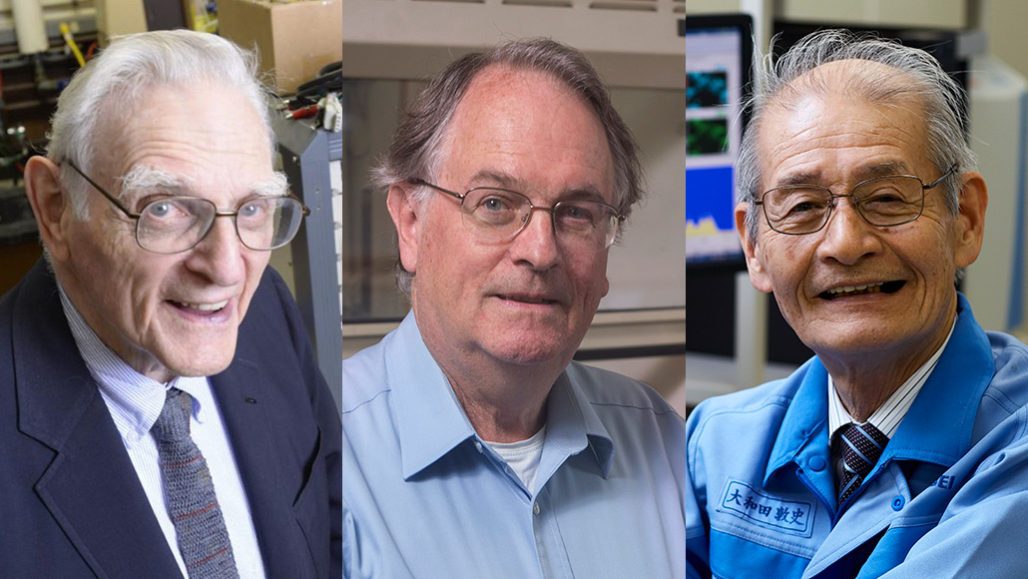
These three scientists, John B. Goodenough, M. Stanley Whittingham and Akira Yoshino (from left), have won the 2019 Nobel Prize in chemistry for their work on lithium-ion batteries.
FROM LEFT: COCKRELL SCHOOL OF ENGINEERING/UNIV. OF TEXAS AT AUSTIN; JONATHAN COHEN/BINGHAMTON UNIV.; HEINZ TROLL/EUROPEAN PATENT OFFICE
By Maria Temming and Jonathan Lambert
Forging the path to a rechargeable world today earned three scientists the 2019 Nobel Prize in chemistry.
Alessandro Volta demonstrated the first electric battery in 1800. Since then, scientists have been working to build better ones. Today’s winners were honored for pioneering the lithium-ion battery. Lightweight and rechargeable, these batteries can be found in everything from portable electronics to electric cars and bicycles. They also provide a way to store energy from renewable energy sources (such as sunlight and wind).
The winners include John B. Goodenough at the University of Texas at Austin. At 97, he is the oldest person to ever receive a Nobel. The other winners are M. Stanley Whittingham and Akira Yoshino. Whittingham works at Binghamton University in New York. Yoshiro works in Japan at Asahi Kasei Corporation in Tokyo and Meijo University in Nagoya.
All three will receive a medal and together split the prize of 9 million Swedish kronor (about $900,000).
Chemist Olof Ramström works at the University of Massachusetts Lowell. He also is a member of the 2019 Nobel Committee for chemistry. He spoke during this morning’s announcement of the prize by the Royal Swedish Academy of Sciences in Stockholm, Sweden. “This battery has had a dramatic impact on our society,” he said. “It’s clear that the discoveries of our three laureates really made this possible.”
The path to a better battery
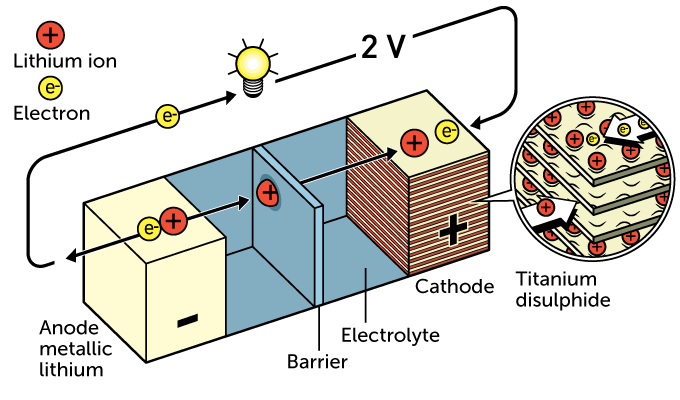
Batteries store electrical energy as chemical energy. All have three main parts. These start with two electrodes: an anode (AN-oad), or negative electrode, and cathode (KATH-oad), or positive one. Separating the two is an electrolyte. This chemical helps electrically charged ions move inside the battery.
Chemical reactions in the anode release electrons. They then travel along a circuit to the cathode. This forms a current.
In the 1970s, Whittingham began experimenting with lithium for his anode. It did not weigh much and readily released electrons and lithium ions. To make a rechargeable battery, he chose titanium disulfide for the cathode. Made of many layers, that cathode stored lithium ions released from the anode. While working with the energy company Exxon, Whittingham used this design for the first lithium battery. It could supply 2 volts.
Goodenough sought to improve on Whittingham’s design over the next decade. His cathode would be made from cobalt oxide. It could sandwich even more ions within its layers. Goodenough’s innovation doubled voltage to 4 volts. (That’s about what it takes to power a modern smartphone.)
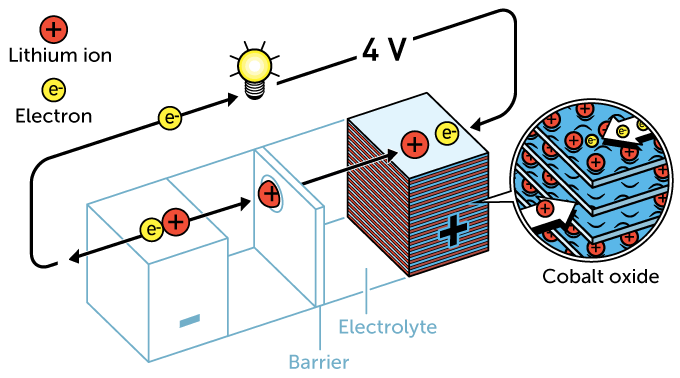
In 1985, Yoshino explored creating an anode from a by-product of oil production. Known as petroleum coke, it was finely layered. And while not made of lithium, it could store lithium ions when charged. Yoshino paired it with Goodenough’s cathode. The result was a safer, more durable, 4-volt rechargeable battery. That design was used for the first lithium-ion batteries to hit store shelves. That was in 1991.
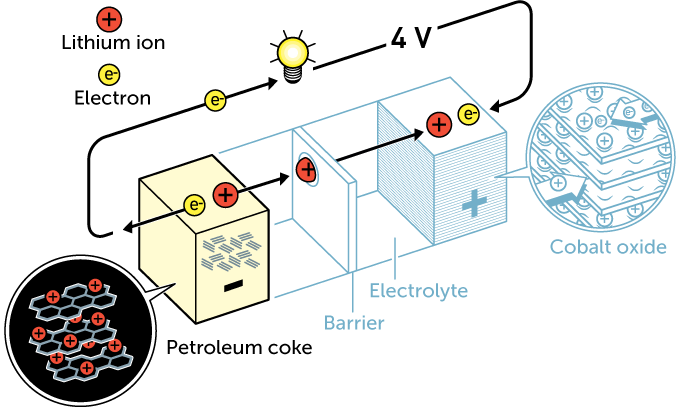
These early devices offered about twice as much energy as the next best batteries. What’s more, they were not one-and-done batteries. These could be drawn down and then recharged hundreds of times before their performance suffered.
Appreciation for the scientists and their work
The Nobel Prize announcement is “really thrilling for the battery community,” says Kelsey Hatzell. She works at Vanderbilt University in Nashville, Tenn. “Stan and Akira and John’s work is so significant.… You can’t imagine going through your daily life without using half a dozen different devices that use lithium-ion batteries.”
At a news conference, Goodenough said “I’m extremely happy that my work has helped people’s ability to communicate.” When asked if he had expected to win, Goodenough replied: “I didn’t expect anything!” He said he’d donate his share of the winnings “to my university to support people who work there.”
Goodenough may not have expected to win, but others had long considered him a shoo-in. “People in the electrochemistry field … have put him number one on our [Nobel prediction] lists for years and years and years and years,” says Amanda Morris. She’s a chemist at Virginia Tech in Blacksburg.
“John is an amazing scientist, with incredible intuition, and a great person,” adds Yang Shao-Horn. She’s a chemist and engineer at the Massachusetts Institute of Technology, in Cambridge. She notes that Goodenough “has inspired generations of scientists and engineers with his positive attitude, honesty and boundless curiosity.”
Lithium-ion batteries now perform much better than those in 1991. Over the past two decades or more, researchers have been working very hard. And it’s paid off. The energy available from these batteries “has doubled — even tripled, in some cases, and the cycle life has improved greatly,” says Shao-Horn. Today, you can recharge these batteries thousands of times. They also have gotten safer and less costly.
Bonnie Charpentier is president of the American Chemical Society. “This is the international year of the periodic table of elements,” she notes. “So it’s fun to have a Nobel Prize that actually names an element.”