Chemists have created a ring-shaped form of carbon
Called cyclocarbon, this molecule is the latest lab-made form of the universal element
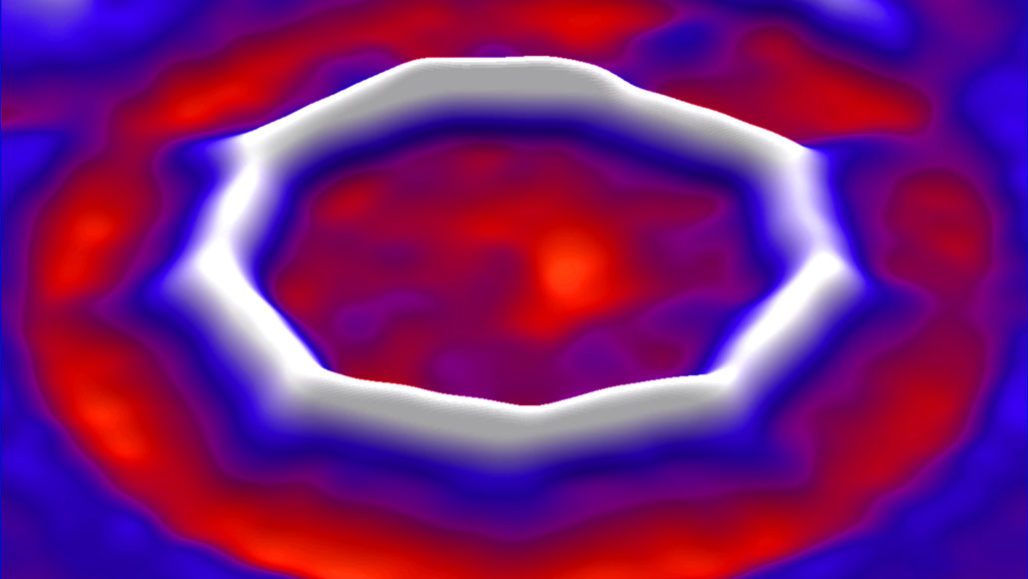
Chemists created a new form of carbon called cyclocarbon. The 18-atom ring is illustrated here using data from an atomic force microscope. Bonds between the atoms are alternately longer and shorter, giving the ring nine sides.
IBM Research
A highly anticipated new form of carbon has finally arrived on the scene.
Called cyclocarbon, this molecule consists of a ring of 18 carbon atoms. Scientists described it online August 15 in Science. It offers a new face to one of chemistry’s most celebrated elements.
“It’s not every day that you make a new form of carbon,” says chemist Rik Tykwinski. He works in Canada at the University of Alberta in Edmonton. Chemists had been trying to create cyclocarbon for a long time. So long that Tykwinski — who wasn’t involved with the new research — had placed a bet about whether it was even possible. He won, he says.
Cyclocarbon joins other molecular forms of the adaptable element, from diamond and graphite to the thin sheets called graphene. There are also tiny spheres known as buckyballs and nano-scale cylinders called carbon nanotubes.
Chemists thought it should be possible to create ring-shaped carbon molecules. But until now, nobody knew what their properties would be, notes physicist Katharina Kaiser. She’s at IBM Research in Zurich, Switzerland. “It’s really amazing that we found it,” she says, “and it’s absolutely great that we could characterize it.”
Kaiser’s team started with molecules of cyclocarbon oxide. These are made of carbon and other atoms. They included groups of carbon monoxide (pairs of carbon and oxygen atoms). Removing the carbon monoxide was a necessary step to create the new ring form of carbon. But that was no easy task. Carbon monoxide helped to stabilize the starting molecule. The researchers managed to pluck off the carbon monoxide groups by zapping the molecule with electricity. They used a specialized tool called an atomic force microscope.
Once the researchers had a bare ring of carbon, they wanted to capture an image of its structure. Again, they used the atomic force microscope. Cyclocarbon reacts easily with other substances — but not table salt. So the team created the new carbon molecule on a salty surface.
Previous research had found hints of cyclocarbon molecules in a gas. But it wasn’t possible to make an image of those molecules. So scientists couldn’t identify the bonds holding the molecule together. Scientists wanted to know if all the bonds were the same length.
The new study resolved that question. It showed that the carbon atoms are held together by alternating long and short bonds.

That should now help scientists refine the computer calculations used to predict the structures of unknown molecules. “There’s still a big question whether many of these … calculations give the right answer,” says Yves Rubin. “So it’s very important to confirm by experiment.” Rubin is a chemist at the University of California, Los Angeles, who also was not involved with the study.
Previous work on new forms of carbon caused great excitement among scientists. The discovery in the 1980s of buckyballs (and the family of molecules that includes them, called fullerenes) won a Nobel Prize. Likewise, the 2004 discovery of graphene won a Nobel. Investigations into its many potential uses in electronics and elsewhere have continued.
But because cyclocarbon isn’t stable, it can’t be bottled up for further study. So, for now, it’s not clear how wide-ranging this new molecule’s impact will be.