Chemists win Nobel Prize for faster, cleaner way of making molecules
Both scientists independently came up with new process — asymmetric organocatalysis
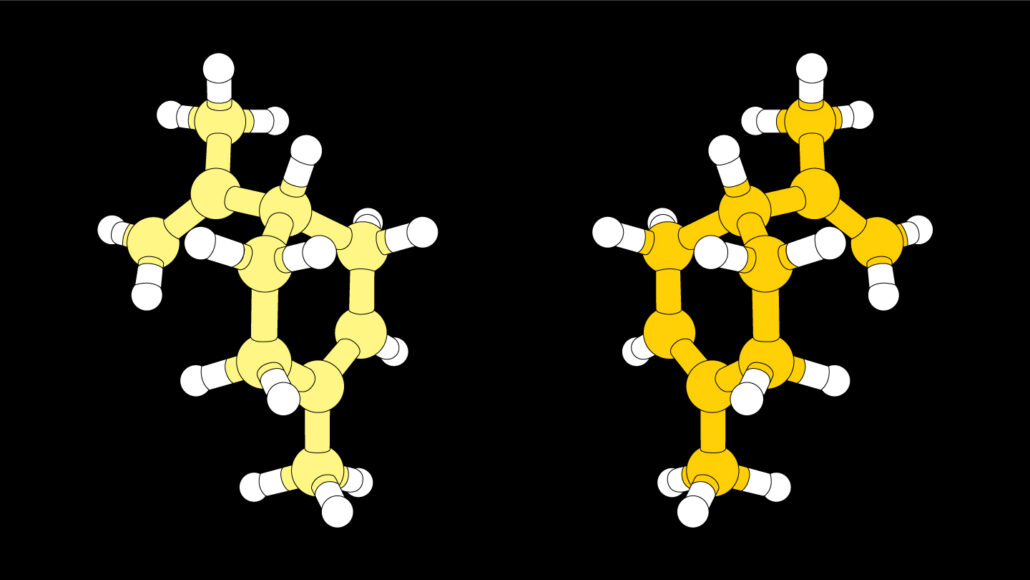
Many chemical reactions produce two versions of a molecule whose structures are mirror images of one another, as the molecule limonene illustrated here. Often, chemists want to make just one of those mirror-image molecules.
©Johan Jarnestad/The Royal Swedish Academy of Sciences
Making molecules is hard work. Atoms must be bonded together in specific arrangements through a series of chemical reactions. Those reactions often are slow and far from straightforward. They also can waste resources. The 2021 Nobel Prize in chemistry goes to two scientists who developed a tool some 20 years ago that revolutionized how chemists create new molecules. Their process is not only faster but also friendlier to the environment.
“This is a fitting recognition of very important work,” says H.N. Cheng. He’s president of the American Chemical Society, based in Washington, D.C. “We can think of chemists as magicians having magic wands in the lab,” Cheng says. “We wave the wand and a reaction goes on.” These Nobel laureates gave chemists “a new wand,” that’s drastically more efficient and less wasteful, he says.
That wand is a new way to speed the reactions that build specific molecules. It’s a process known as asymmetric organocatalysis (AY-sih-MEH-trik Or-gan-oh-kah-TAL-ih-sis). This year’s winners came up with the idea for it independently. One of the chemists, Benjamin List, works at the Max Planck Institute for Coal Research. It’s in Mülheim an der Ruhr, Germany. The other is David MacMillan. He works at Princeton University in New Jersey.
Making new drugs or designing novel materials often requires assembling simple chemical building blocks to form new molecules. But these chemical building blocks can’t just be thrown together. Instead, they must be carefully combined through a step-by-step series of processes. Many of these procedures create two versions of a molecule, ones that that are mirror images of each another. And often those left- and right-handed versions can have very different effects.
For example, thalidomide (Thah-LID-uh-myde) is a drug prescribed in the 1950s and ‘60s to prevent morning sickness in early pregnancy. But it caused birth defects in more than 10,000 babies. The problem: This drug supplied both mirror-image forms of the molecule, and one of them was toxic.
It was a painful lesson for chemists. Today, building such asymmetric molecules and controlling which version gets produced is extremely important. That’s especially true for medicines.
The role of catalysts
Chemical reactions can be coaxed to take place using catalysts. These molecular workhorses speed up those reactions without being transformed by them.
Chemists have long known about two kinds of catalysts: enzymes and metal complexes. Enzymes are big, clunky proteins. Through evolution, organisms have developed enzymes that perform very specific chemical actions in the body. Many can be reproduced in the lab. But making them on a large scale can be hard. Metals, such as platinum or cobalt, can kick-start some reactions too. But many only work in airless, waterfree conditions. And they can be hard to achieve in manufacturing plants. What’s more, many metal catalysts are toxic and costly.
For much of history, these were the only tools chemists had to make new molecules. “But in the year 2000, everything changed,” says Pernilla Wittung-Stafshede. She’s a chemist at Chalmers University of Technology in Gothenburg, Sweden. She’s also a member of the Nobel Committee for Chemistry, which selected this year’s winners.
Back in 2000, Benjamin List worked at the Scripps Research Institute. It’s in La Jolla, Calif. He was studying a reaction used to link two organic molecules together through bonds in their carbon atoms. In organisms, such reactions are key to converting food into energy. And they depend on a large and complex enzyme called aldolase A.

Only a small part of this enzyme actually catalyzes the reaction, however. List discovered that one amino acid — proline — could do the work of this big clunky protein. And by using proline, chemists could make far more of one of the mirror-image final products.
“When I did this experiment, I didn’t know what would happen,” List said at an October 6 news conference. “I thought maybe it’s a stupid idea.” But when it worked, he now recalls, he realized “it could be something big.”
At about the same time, MacMillan was working at the University of California, Berkeley. He was focusing on another chemical reaction — one that forms rings of carbon atoms. It’s an important reaction. Chemists use it all the time to make products as different as rubber and medicines. While it works, it tends to be very slow. And it relies on finicky metal catalysts that won’t work when wet.
So MacMillan designed small carbon-based molecules — organic molecules — that mimicked the metals’ catalytic action. They also worked more simply. And these, too, favored the production of one of two possible mirror-image forms of the final product. MacMillan coined a term for this process: asymmetric organocatalysis.

The value of this research
List’s and MacMillan’s work prompted others to seek out more organic catalysts and to study how they might be used. These catalysts tend to be small carbon-and-hydrogen molecules which might also include oxygen, nitrogen, sulfur and/or phosphorus.
Catalysis is a big deal. Roughly one-third of the world’s collective income depends on it, notes Peter Somfai. He’s a chemist at Lund University in Sweden and another member of the Nobel Committee for Chemistry. At an October 6 news conference announcing the new winners, he noted “We now have a new powerful tool available for making organic molecules.” He said it’s one that can be drastically more efficient and “greener” than previous methods.
To highlight how much more efficient this process is, Somfai pointed to strychnine (STRIK-nyne). The toxic molecule is made by certain plants and is sometimes sold as a rat poison. Its complex structure makes it a good benchmark for comparing how efficiently chemicals can be made using different processes in the lab or by a company. Chemists once had to use an extremely wasteful process to make the compound. There were 29 different chemical steps. And in the end, just 0.0009 percent of the initial raw materials ended up as strychnine. But through organocatalysis, strychnine can now be made in just 12 steps. In fact, Somfai said, this process is 7,000 times more efficient than the old one.
And because this process eliminates use of toxic chemicals, it’s also a far more environmentally friendly process.
If building new molecules is like playing chess, asymmetric organocatalysis has “completely changed the game,” Somfai said. “It’s like adding a new chess piece that can move in different ways.”
For their achievements, List and MacMillan will each get a medal and share 10 million Swedish kroner (more than $1.1 million).