Converting trash to valuable graphene in a flash
This so-called flash graphene can strengthen other materials — and fight climate change

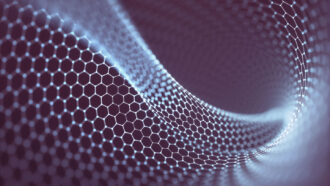
Here, electrical energy is being routed into a batch of carbon, reformulating almost all of the bonds between carbon atoms at once, to make graphene. The excess energy appears as the visible flash.
Jeff Fitlow/Rice University
Plastic, food wastes, tires and coal all have one thing in common: They contain lots of carbon. When those items break down or burn, they release that carbon into the atmosphere as carbon dioxide or methane. Those heat-trapping gases contribute to a warming of Earth’s atmosphere. But scientists in Texas have discovered a way to convert anything with carbon — even trash — into graphene (GRA-feen). This process keeps carbon out of the atmosphere. What’s more, the graphene can be added to other materials to make them “greener.”
The team published its findings on January 27 in Nature.
Graphene consists of a flat layer of carbon atoms just one-atom thick. Each atom bonds to three other carbon atoms. This creates a honeycomb-like grid. A single sheet of graphene is the thinnest material on Earth. It’s also the strongest.
Scientists have been testing graphene for use in all types of materials. These include fabric, hair dye, electronics and super-tall towers. And graphene will strengthen existing materials, notes James Tour. He is an organic chemist and nanotechnology specialist at Rice University in Houston, Texas. Tour and applied physicist Duy Luong, who also works at Rice, led the new study.
Although graphene has huge potential, it has faced some problems. It’s proven hard to make more than just a tiny amount at one time. The usual methods tend to create multiple layers of graphene that are tough to separate. And the end product can be quite expensive — up to $200,000 per ton. That’s why chemists have been searching for a cheap way to make large amounts of single-layer graphene.
A flash of inspiration
Luong had used a laser to make graphene before. He zapped carbon-rich materials to rapidly — and briefly — heat them. That flash of heat caused carbon atoms to rearrange themselves into layers of graphene. Luong quickly found that “Anything [with carbon] can be converted into graphene if it is heated up hot enough and fast enough.” That was an important first step.
He then turned up a paper on research in another lab. It had used a process called flash joule heating to create particles on a billionth-of-a-meter scale. (A joule is a unit of energy.) This flash process superheated materials with electricity, not a laser.

Those two observations got Luong wondering: Could flash joule heating turn everyday wastes into graphene? To find out, he set up an experiment.
Luong started with a very-carbon-rich material. Called carbon black, it’s what’s left behind after burning wood or heavy (viscous) petroleum. Luong placed some powdered carbon black inside a tube made from quartz. Then he stuffed copper wool into each end to press the powder together. He poked brass screws into each end of the tube. These acted as the electrodes of a capacitor. (That’s a battery-like device.) The electrodes were connected to the rest of the setup. This safely supplied power.
Then Luong flipped the switch to turn on the electricity. At once, the carbon black flashed a blinding white. And when Luong poured the carbon from the tube, it was no longer a powder. Instead, it came out in clumps.
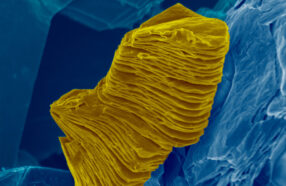
He examined them closely. The atoms had rearranged themselves into a clear honeycomb pattern. It was graphene! Even more exciting: It didn’t have lots of tightly stacked layers. The many layers were arranged loosely. When Luong mixed them with water and a surfactant (Sur-FAK-tunt), they separated with ease. (A surfactant helps materials mix with water.)
Luong found that the critical temperature needed to make flash graphene is just over 2,700° Celsius (almost 5,000° Fahrenheit). Triggering this carbon transformation took just a 10-millisecond flash of electricity.
A way to tackle climate change?
Flash joule heating turned carbon black into pure graphene. But would it do the same for anything with carbon in it? To find out, Luong tested the setup with coal and coffee grounds. Both tests converted 80 to 90 percent of the carbon into pure graphene. Non-carbon atoms simply vaporized at the high temperature, Luong says. (He didn’t capture that vapor. But, he notes, it could be collected and used for other purposes.)
Luong believes this flash graphene could have a huge impact on how we make things. It “can be put back into plastic,” Luong says. That would strengthen the plastic, cutting how much would be needed for any application. To test this, he and his coworkers added a bit of flash-graphene to a plastic polymer. It more than doubled the strength of the polymer. And that was when the added graphene made up only a tenth of a percent of the polymer.
They added even less graphene to cement — just a twentieth of a percent by weight. After letting the cement cure for 28 days, it now was at least 25 percent stronger than graphene-free cement. In all of these products, adding graphene would reduce the overall amount of plastic or cement that would be needed for some application.
What’s more, adding graphene to products traps carbon atoms in these solid objects. Left to decompose, trash and recycled goods break down into methane or carbon dioxide. Both act as greenhouse gases. By trapping the sun’s heat close to the ground, those gases work to warm Earth’s climate. Adding graphene — especially that made from trash — to materials should cut greenhouse-gas releases and help slow that rate of warming.
Now they need to scale up
Luong’s experiment created up to one gram of easy-to-separate graphene in a single flash. That’s more than any other experiment has been able to produce. Next, he plans to make graphene in larger amounts. Eventually, he wants to make the process work on a massive scale. If he succeeds, it could turn food and plastic wastes, as well as coal and used tires, into a valuable manufacturing ingredient. At the same time, it could reduce society’s carbon footprint. (That’s the amount of carbon dioxide and methane our everyday actions release into the atmosphere.)
This innovation could work like King Midas’s golden touch, says Zhiwei Peng. He’s a graphene researcher at the University of Maryland in College Park who was not involved in the new study. “In the blink of an eye,” he says, you can turn “used plastic wastes and discarded food into ‘black gold’ — graphene.” And all it would cost to make a single gram is enough energy to light a 60-watt bulb for two minutes, he says.
Luong wants teens to know that high school classes can provide the training needed to make big discoveries. He created his working electrical system thanks to classes he took when he was younger. That’s when he learned about building circuits. Now he says he can use what he learned back then “for a greater purpose.”
This is one in a series presenting news on technology and innovation, made possible with generous support from the Lemelson Foundation.