Explainer: In chemistry, what does it mean to be organic?
At a minimum, these molecules need carbon. But that’s only the first requirement

Carbon can become part of vast numbers of molecules, including many that make up plant and animal tissues. That’s one reason these molecules are part of the chemistry of life.
ipopba/iStock /Getty Images Plus
Out of 118 elements, only one has its own field of study: carbon. Chemists refer to most molecules that contain one or more carbon atoms as organic. The study of these molecules is organic chemistry.
Carbon-based molecules get special attention because no other element comes close to carbon’s versatility. More types of carbon-based molecules exist than all non-carbon ones put together.
Scientists generally define a molecule as organic when it contains not only carbon, but also at least one other element. Typically, that element is hydrogen, oxygen, nitrogen or sulfur. Some definitions say that a molecule must contain both carbon and hydrogen to be organic.
(By the way, in farming, “organic” refers to crops grown without certain pesticides and fertilizers. That use of “organic” is very different from the chemical definitions here.)
Living things are built with organic molecules and operate using organic molecules. Indeed, organic molecules perform the tasks that makes a living thing “alive.”
DNA, the molecular blueprint for our bodies, is organic. The energy we get from food comes from breaking down carbon-based — organic — molecules. In fact, until the 1800s, chemists thought that only plants, animals and other organisms could make organic molecules. Now we know better. Our oceans created organic molecules before life even existed. Organic molecules can also be made in the lab. Most medicines are organic. So are plastics and most perfumes. Still, organic molecules are seen as a defining feature of life-forms.
But living things also contain lots of molecules that are not organic. Water is a good example. It makes up about six-tenths of our bodyweight but is not organic. We must drink water to live. But drinking water doesn’t satisfy hunger. A hamburger or beans, for instance, contains those organic molecules needed to fuel our bodies’ growth.
In living things, organic molecules usually fall into one of four categories: lipids (such as fats and oils), proteins, nucleic acids (such as DNA and RNA) and carbohydrates (such as sugars and starches). These molecules can get big, though still too small to see with just our eyes. Some may even be organic molecules bonded to other organic molecules. The big ones, made by linking a lot of littler ones, are known as polymers.
Carbon: Molecule-maker supreme
Three things make carbon special.
- Covalent bonds are those within a molecule where various atoms share an electron. Those tight linkages hold the atoms close to one another. Each carbon atom can form four covalent bonds at once. That’s a lot. And it’s not just that carbon can form four bonds, but rather that it wants to form four bonds.
- Carbon’s covalent bonds come in three types: single, double and triple bonds. A double bond is extra-strong and counts as two of carbon’s four desired bonds. A triple bond is stronger still, and counts as three. All these bonds and bond types allow carbon to make many types of molecules. In fact, simply replacing any single bond with a double or triple bond will give you a different molecule.
- Carbon atoms tend to link up with other carbon atoms to form chains, sheets and other shapes. Scientists call this ability catenation (Kaa-tuh-NAY-shun). Plastic is the name for a family of organic polymers. Their long carbon chains can either be straight or branch out like trees. Each trunk or branch of these polymers is made from a backbone of catenated carbons. Carbon can link into ring shapes, too. Caffeine, a molecule in coffee, is a compact, two-ring, spider-shaped molecule held together by the catenation of carbon atoms. Carbon atoms even connect to form perfectly spherical 60-carbon balls. These are known as buckyballs.
Hydrocarbons: The basis of fossil fuels
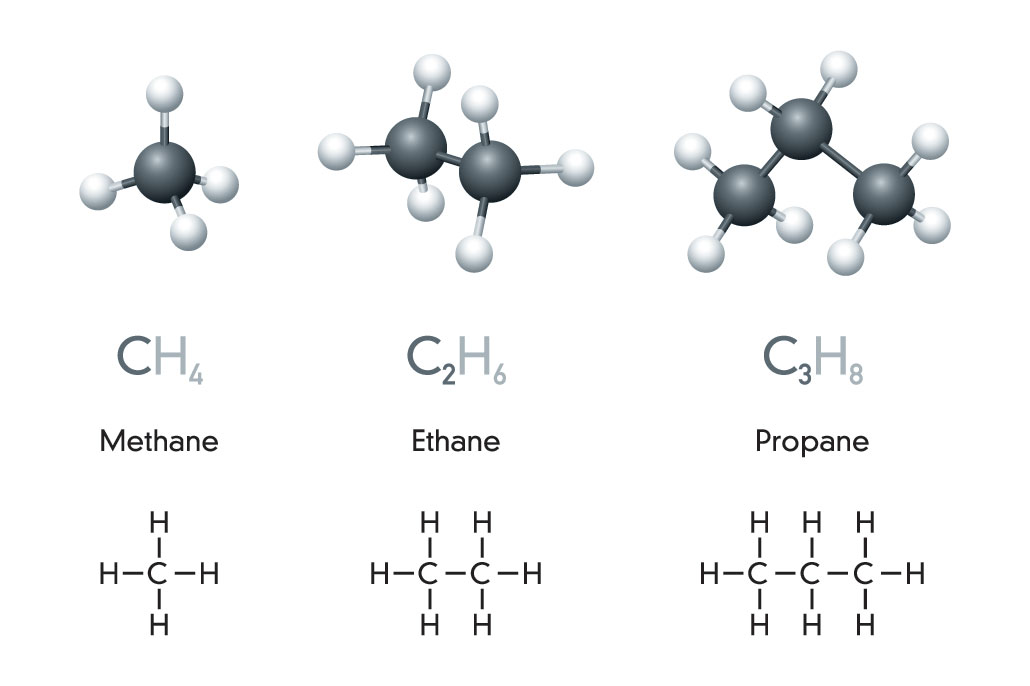
Crude oil and natural gas are fossil fuels made from a complex mix of natural organic chemicals, generally known as hydrocarbons. That term is a mash-up of hydrogen and carbon. These molecules are, too.
The simplest hydrocarbon is methane (METH-ain). It’s made from a single carbon atom bonded (covalently) to four hydrogen atoms. A two-carbon version, ethane (ETH-ain), holds onto six hydrogen atoms. Add a third carbon — and two more hydrogens — and you get propane. Notice that the end of each name stays the same. Only the first part, or prefix, changes. Here, that prefix tells us how many carbons the molecule holds. (Peek at the back of a bottle of hair conditioner. Try to spot some of these prefixes hidden in the long chemical names.)
Once we reach four bound carbons, new hydrocarbon shapes become possible. Since carbon chains can branch, four carbon atoms (and their hydrogens) may bend and connect into unusual shapes. That results in new molecules.
Beyond hydrocarbons
Even more molecules become possible when something else stands in for one or more of a hydrocarbon’s hydrogen atoms. Based on which atom takes hydrogen’s place, scientists can predict how the new molecule will act — even before it’s been tested.
For example, having just carbon and hydrogen atoms, a simple propane molecule won’t dissolve in water. It will be hydrophobic (Hy-droh-FOH-bik). That means water-hating. The same is true for other oils made of hydrocarbons. Try this: Pour canola oil into water. Watch the oil layer float atop the water. Even if stirred, the oil won’t mix.
But if a scientist replaces a few of the hydrogens in those molecules with a bound pair of oxygen and hydrogen atoms — known as a hydroxyl (Hy-DROX-ull) group — the molecule suddenly dissolves in water. It has become water-loving, or hydrophilic (Hy-droh-FIL-ik). And the more hydroxyls added, the more water-soluble the former oil becomes.
So what is inorganic?
Not all carbon-based molecules are organic. Some, such as carbon dioxide (or CO2), can be “inorganic.” The lack of hydrogen is why many chemists classify carbon dioxide this way. To be “organic,” these chemists argue, a molecule must combine its carbon with some hydrogens.
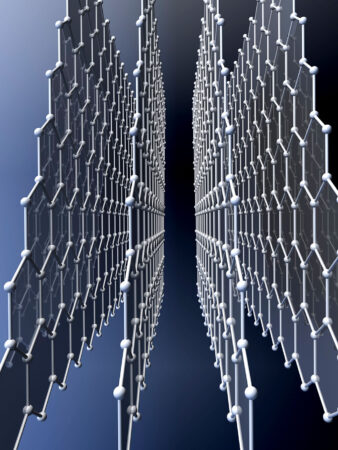
Diamonds are also inorganic. They are made solely of carbon atoms. So is graphene. (When stacked in sheets, graphene becomes graphite, the soft black stuff found inside pencils.) Diamond and graphene are made of the same atoms, just arranged differently. Diamond’s carbon atoms connect up, down, and sideways to form three-dimensional crystals. Graphene’s carbon forms sheets that stack like paper. But the size of those sheets is not standard; it depends solely on the amount of carbon used.
Most scientists argue that diamond and graphene are inorganic carbon because neither graphene nor diamond counts as a molecule. At least, not in the strict sense of the word. Molecules should be discrete assemblies of atoms. And though there are endless types of molecules, each type should “have a fixed molecular weight,” explains Steven Stevenson. He’s a chemist at Purdue University Fort Wayne in Indiana.
A true molecule has a fixed weight because it contains a specific number of atoms that are combined in a particular way. Diamond contains atoms arranged in a specific way — but not a specific number of atoms. Big diamonds have more atoms than small diamonds. So diamond isn’t a true molecule, Stevenson says.
Sugar, on the other hand, is a molecule. And it’s organic. A cube of sugar may look diamond-like. But inside, sugar contains bazillions of separate sugar molecules all stuck together. When we dissolve sugar in water, all we do is unstick those true molecules.
And then there are the fullerenes
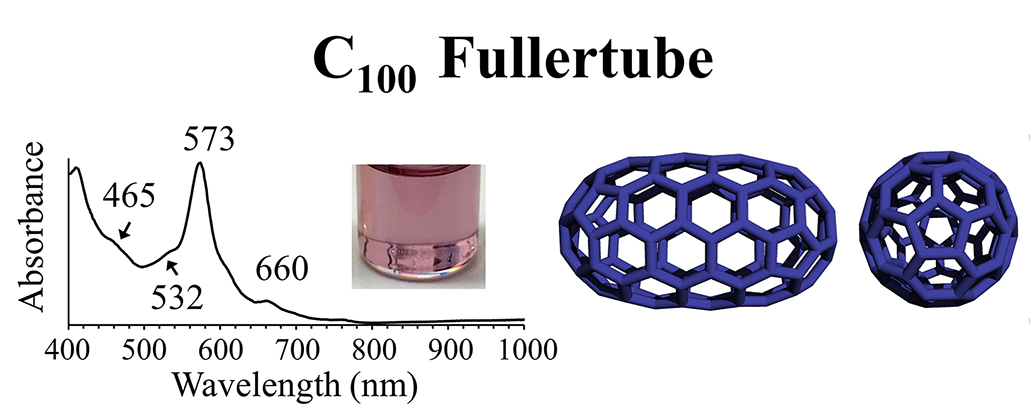
True molecules made entirely of carbon do exist. Known as fullerenes, these all-carbon molecules come in a variety of shapes, such as buckeyballs and tubes. Are these organic?
“I think it depends on which organic chemist you ask,” Stevenson says. He’s a fullerene specialist. In 2020, his lab discovered a new family of these molecules called fullertubes. Stevenson refers to the 100-carbon version as simply C100. It shows a notable hue. “I cannot tell you how nice that is,” he recalls, to suddenly realize “you’re the first one in the world to know that this new molecule is purple.”
Fullertubes count as molecules. But are they organic?
“Yes!” Stevenson argues. But he also acknowledges that some chemists would disagree. Remember, many typically define organic molecules as not just having carbon, but also hydrogen. And the new fullertubes? They’re just carbon.