Explainer: How is water cleaned up for drinking
Treatment plants focus on removing germs and debris, often overlook industrial chemicals

Most people get their drinking water from lakes, rivers or groundwater aquifers. But before it flows out the tap, cities will typically treat the water in big industrial plants, as here, to purify it.
WangAnQi/iStockphoto
People are used to turning the handle on a sink and seeing a stream of clear water pour forth. But where does this water come from? Typically, a town will pump it from a river, lake or groundwater aquifer. But this water can host an array of germs and solids — waterborne dirt, rotting plant bits and more. That’s why a community will typically process that water — clean it — through a series of steps before sending on to your faucet.
The steps of water treatment
The first step is usually to add coagulants (Koh-AG-yu-lunts). These are chemicals that cause those solid bits to clump together. Even if those solids didn’t hurt you, they could cloud water and give it a funny taste. By making these bits clump, they become bigger — and easier to remove. A gentle shaking or spinning of the water — called flocculation (FLOK-yu-LAY-shun) — helps those clumps to form (1).
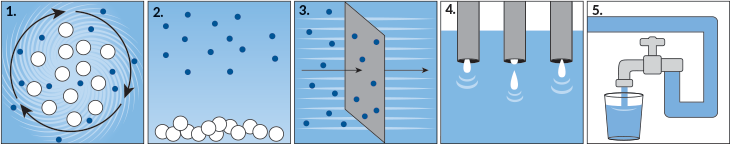
Next, the water flows into big tanks where it will sit for a while. During this settling period, the solid sediments begin to fall to the bottom (2). The cleaner water atop it then moves through membranes. Like a sieve, they filter out smaller contaminants (3). Then the water is treated with chemicals or ultraviolet light to kill harmful bacteria and viruses (4). Following this disinfection step, the water now is ready to flow through pipes to homes throughout a community (5).
Different communities may tweak this process in some way. They may add chemicals at different stages to trigger reactions that break down chunky, toxic organic molecules into less harmful bits. Some may install an ion-exchange system. This can separate contaminants by their electric charge to remove ions. These include magnesium or calcium, which can make water “hard” and leave a scaly deposit on faucets and pipe. It may also take out heavy metals, such as lead and arsenic, or nitrates from fertilizer runoff. Cities mix and match different processes. They also vary the chemicals used, based on the qualities (chemical recipe) of the incoming local water.
Some water companies are streamlining their treatment process even more by installing technologies such as reverse osmosis (Oz-MOH-sis). This technique removes nearly every contaminant in water by forcing the water molecules through a selectively permeable membrane — one with really tiny holes. Reverse osmosis can replace a number of steps in the water treatment process or reduce the number of chemicals added to water. But it’s expensive — out of reach for many cities.
Well owners are on their own
More than one in every seven U.S. residents gets water from wells and other private sources. These are not regulated by a federal law known as the Safe Drinking Water Act. These people face the same contamination challenges as municipal water systems. The difference, individual families have to worry about their own cleanup and treatment — without help or funding from other community members.
“When it comes to lead in private wells … you’re on your own. Nobody is going to help you,” says Marc Edwards. He’s the Virginia Tech engineer who helped uncover the Flint, Mich., water crisis. Edwards and Virginia Tech colleague Kelsey Pieper collected water-quality data from more than 2,000 wells across Virginia in 2012 and 2013. Some were fine. Others had lead levels of more than 100 parts per billion. When levels are higher than EPA’s 15 ppb threshold, the government requires that cities take steps to control corrosion and notify the public. Homeowners are unlikely to ever realize they have such a problem with their own well. The researchers reported those findings in 2015 in the Journal of Water and Health.
To remove lead and other contaminants, well users often rely on point-of-use treatments. This is usually some type of filter. It’s placed at or near the faucet to remove most — but not all — pollutants. Some people may spring for the gold-standard treatment at home: a costly reverse osmosis system.