Germs power new paper batteries
These ‘papertronics’ power systems may be especially useful in remote or dangerous places, scientists say

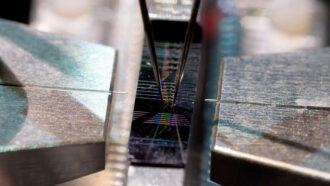
What looks like a folded piece of a paper is actually a battery powered by bacteria.
Seokheun "Sean" Choi
Engineers in upstate New York have invented a folded paper device that looks like a decorated art project. But don’t be fooled. This is actually a paper-based battery. No, it doesn’t look like any of those metal batteries running flashlights or smartphones. This alternative to electronics is based on paper. It represents a step forward in the field of papertronics (short for paper electronics). In these systems, the battery can be printed on a page. Well, most of it can: The battery’s power consists of living bacteria.
Paper electronics are simple to make and inexpensive, notes study leader Seokheun Choi. He’s an engineer at Binghamton University, part of the State University of New York system. These batteries also would be flexible and disposable, he adds. And powered by germs, they need no electrical outlet to recharge. They just need more bacteria, which can be found everywhere — including in dirty water.
Most batteries use chemicals to generate electricity. Substituting bacteria can be an advantage, Choi says. “They are cheap, self-repairing and self-maintained,” he notes. What paper-based batteries won’t do is generate much power. They do, however, create enough to run small devices in faraway or dangerous places — such as a battlefield. They might also find use in medicine. For instance, they might power tiny sensors, such as the types used to measure blood sugar.
Choi and Yang Gao, also at Binghamton, describe their new invention in the January 2017 issue of Advanced Materials Technology.
Such devices are based on an observation made more than a century ago — that microbes produce a trickle of electricity as they digest food. Scientists refer to the bio-batteries based on this principle as microbial fuel cells.
A fuel cell generates electricity like a regular battery. But a regular battery stops producing electricity when its internal chemical reactions stop. A fuel cell uses fuel that can be replenished. In this case, bacteria serve as the fuel. By replenishing more germs, as needed, scientists can keep these fuel cells running.
Papertronics’ advantages
Ordinary electronics often contain toxic materials. Paper-based bacterial batteries may offer a safer choice, says Derek Lovley. A biologist, he works on bacteria and batteries at the University of Massachusetts, Amherst. A battery powered by germs may never run out of juice. “It can go on forever,” he says, as long as the bacteria have enough to eat.
Ordinary batteries convert chemical energy into electrical energy. They have three main parts. One is the anode (AN-ode). It produces negatively charged particles called electrons. (Flowing electrons make electricity.) Another is the cathode (KATH-ode). It receives electrons from the anode. The third is a chemical electrolyte. It is usually found between the anode and the cathode. Chemical reactions among the materials cause electrons to leave the anode. They travel along a conductor to the cathode. From there they can move on to power a connected device.
In the battery made by Choi’s team, the scientists used wax to hold everything in place. The wax also made the paper hydrophobic. (That means water won’t soak in and weaken it.) Their anode was an electricity-conducting material painted on one side of the paper. Silver, sprayed onto the paper’s bottom, provides the cathode. The anode and cathode are separated by the wax and paper. Choi says that paper layer also acts like a small container where the bacteria can dwell.
Here’s how: To power the battery, a user adds water to the reservoir that contains bacteria and organic compounds. (Organic compounds contain carbon. Choi says a simple sugar, such as glucose, would be a good choice here.) As bacteria digest their meal, they release electrons. Those electrons pile up at the anode. Then, when a device or wire connects to the anode and cathode, the electrons flow to the cathode. To recharge the battery, someone need only add water hosting more bacteria. Even wastewater would work, Choi says.
In past projects, scientists have shown that it’s possible to print metal or other materials on paper. That means they can print circuits — even parts of a battery. Those manufacturing techniques could be used for Choi’s paper-based battery, too.
Lab tests have shown that the new battery can produce a trickle of a current. Now, Choi and his team are looking at ways to increase the power. They’re studying different shapes and materials for the anode and cathode. They’re also looking for the best ways to combine batteries for more power.
“The beauty of the paper devices is that you can simply stack them or fold them to connect them,” he says.
This is one in a series presenting news on technology and innovation, made possible with generous support from the Lemelson Foundation.