Hairy nanoparticles put viruses in a deadly embrace
The discovery could lead to teeny-tiny treatments for a wide range of diseases
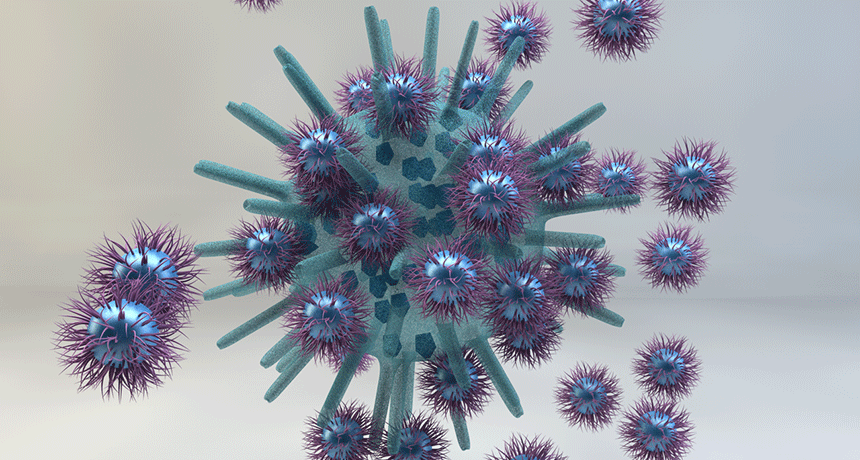
In this artist’s illustration, gold nanoparticles (bright blue) covered in tiny hairs (shown in purple) surround a virus and attach to it. As more hairs attach, they squeeze the soft surface of the virus to find places to stick. That squeezing builds enough pressure to squash the virus.
Francesco Stellacci
By Ilima Loomis
Our tiniest enemies are some of the hardest to fight. The viruses that infect people with influenza and HIV kill millions of people each year. Doctors still don’t have good weapons to quash the germs responsible. Most common viruses, such as those that cause colds, can’t even be treated with drugs. Drugs that do exist work against just a few types of viruses. And they only weaken these germs; they don’t destroy them. Now scientists have figured out how to slay many types of harmful viruses. Their new weapon of choice: nanoparticles.
Francesco Stellacci is a materials scientist who works in Switzerland at the École Polytechnique Fédérale in Lausanne. Some nanoparticles that he worked with appeared similar to a protein that sticks to viruses. And that was his inspiration. He suspected that if he could create a new nanoparticle that worked this way, it would grab onto a virus and not let go.
The particle he created is just five nanometers wide. For perspective, a human hair is 2,000 times that width. The particle is made from a tiny crystal of gold. A forest of even tinier threads cover its surface. Those threads are made from carbon-based compounds.
Stellacci’s group tested these tiny particles against a broad range of viruses. And they now report success.
The nanoparticles destroyed common germs like the herpes virus and human papilloma virus. The first triggers a range of symptoms, including cold sores in the mouth. The second is responsible for the most common type of sexually transmitted infection — one that can cause genital warts and even cancer. The hairy spheres also quashed respiratory syncytial (Sin-SISH-ul) virus. It infects the lungs, causing cold-like symptoms — except in babies and the elderly. It can leave them with life-threatening pneumonia.
Finally, the new nanoparticles killed dengue (DEN-gay) virus. It infects some 400 million people each year. There has been no treatment for this tropical illness, which is spread by mosquitoes. Symptoms include severe eye, muscle and joint pain; vomiting; bleeding gums; difficulty breathing and black tarry stools.
Clearly, the new nanospheres appear to hold a lot of promise.
Stellacci’s team described them and how they work in the February Nature Materials.
A physical attack
To reproduce, a virus must enter the body’s cells. There it starts to hijack those cells to make many copies of itself. Afterward, the infected cell bursts. This releases a legion of new viruses that can spread to other cells in this person or others.
Today’s antiviral drugs work by stopping a virus from multiplying after it enters a healthy cell. They defeat the virus — but only temporarily. If the patient stops taking the drug, the virus can return. And because these drugs attack viruses using chemicals, they can sometimes produce toxic side effects.
“Viruses are made from similar things [to what] we are made of,” Stellacci explains. “So if you want to chemically damage them, you will damage the host cells, too.”
His group’s nanoparticles stop the germs differently. First, they attack a virus before it enters a healthy cell. Then, instead of fighting it with toxic chemicals, these hairy balls begin a physical attack. Those hairs on the outside of a nanoparticle grab onto the virus with an unbreakable bond. Then they apply pressure. Eventually, Stellacci says, “That pressure blows up the virus.”
How do tiny nanoparticles put the squeeze on a virus that is many, many times their size? It comes down to those hairs.
These attach to tiny physical structures on the outside of a virus known as receptors. Receptors are spaced much farther apart than the hairs. So as more and more hairs attach, they gradually pull on the receptors. This brings them closer and closer together, squeezing the germs’ soft surface. The pressure of this big squeeze builds, especially if many nanoparticles grab on. Apply enough pressure — and pop! The virus is a goner.
Hairy helpers
Scientists have been studying nanoparticles for a long time. But they’ve only recently begun learning how to use them in medicine, says Alexander Spokoyny. He’s a chemist at the University of California, Los Angeles. Stellacci combined properties that nanoparticles already had and used them in an innovative, new way, Spokoyny says.
“If you can take some relatively simple chemistry and learn how to control it, you can create very complex assemblies that potentially solve a very important problem,” he says.
Because the nanoparticles were already covered with hairs, Stellacci says, it was easy to adapt them to fight viruses. But now that scientists know how well they work, he said it should be possible to engineer new types of particles to do the same thing.
Stellacci’s team has already started testing the nanoparticles in animals. That’s the next step toward someday turning them into a drug to treat people. He also hopes other scientists will become inspired with their own ideas on how to turn his discovery into medicine that might save lives.
“I’m trying to explain to the community that there is a new way of fighting infection,” he says.
This is one in a series presenting news on technology and innovation, made possible with generous support from the Lemelson Foundation.