New cloth cools you when you’re hot, warms you when you’re cold
3-D printing makes this “phase-change” fabric, which has even more new tricks

Sweat evaporates from this firefighter, carrying heat away from his skin, which cools him down. This process of sweat’s water changing from liquid to vapor is one common example of a “phase-change.” Firefighters are among workers who might appreciate the new fabric.
kali9/iStock/Getty Images Plus
Imagine if the same jacket that warms you up on chilly days would also cool you down on hot ones. Fabrics with “phase-change” properties can do that. And a research team from China now shows that 3-D printing techniques can yield a strong phase-change cloth — one that hides some more tricks up its sleeve.
This new fabric not only helps regulate temperatures, but also conducts electricity. It even resists the radio waves used in Wi-Fi.

“The combination of those properties is what’s very intriguing,” says Sergio Granados-Focil. He did not take part in the cloth’s development. But this polymer chemist at Clark University in Worcester, Mass., is familiar with phase-change materials.
To understand the fabric’s phase-change properties, consider the DC Comics’ Metamorpho. This superhero could — poof! — suddenly phase-change his solid body into a gas. Or, when he needed it, become a liquid.
Metamorpho is fictional. But phase-change is very real. Water goes through a phase-change when it freezes or evaporates. In each case, it’s the same molecule — just in different chemical phases. The removal or addition of heat triggers the change between phases. Imagine ice cubes melting into a glass of water. The ice absorbs the water’s heat. That warms up the ice, but cools down the water.
Phase-change cloth also works by moving heat around. The Chinese team trapped a phase-changing polymer inside the fabric. It changes between a crystal and a non-crystal form. Becoming a crystal absorbs — or removes — body heat. That will cool you down. But this heat isn’t gone. The fabric stores it. And the polymer will release that heat when it shifts back into a non-crystal. This now warms you up.
The new fabric contains a novel mix of ingredients. In developing it, says Yongyi Zhang, “for the first time we demonstrate a scalable and controllable 3-D printing strategy.” Zhang studies nanotechnology at the Chinese Academy of Sciences in Suzhou.
His team described its innovative fabric in the January 31 ACS Applied Materials & Interfaces.
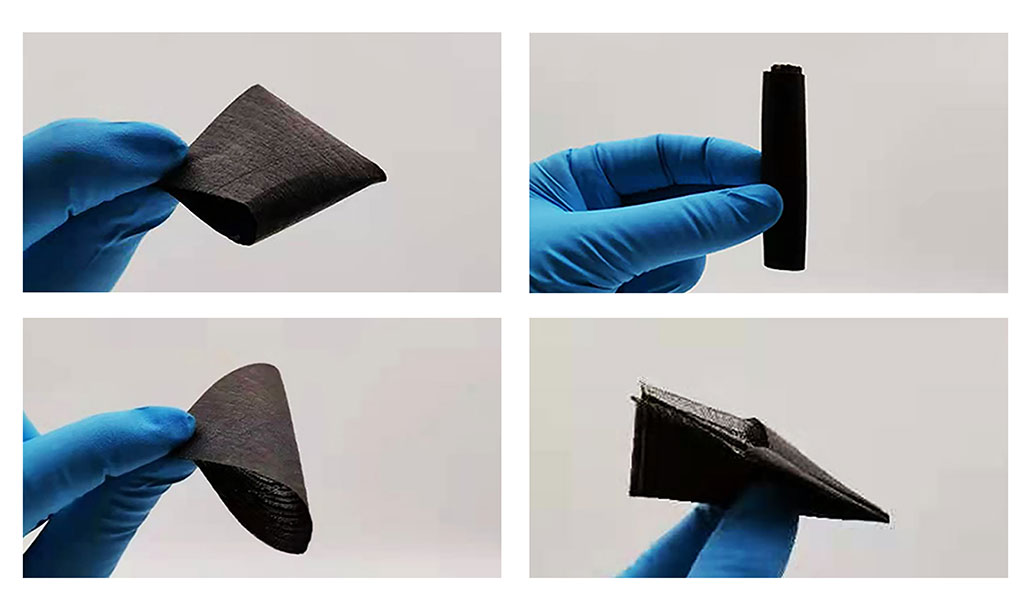
Shape is key to the polymer’s magic
The new fabric’s polymer will change its shape at different temperatures. That’s the phase-change part. In the non-crystal phase, “the polymer chains can move around each other,” notes Emily Pentzer, who did not take part in the new work. This polymer re-crystallizes again as it cools, the polymer scientist explains. Pentzer works at Texas A&M University in College Station.
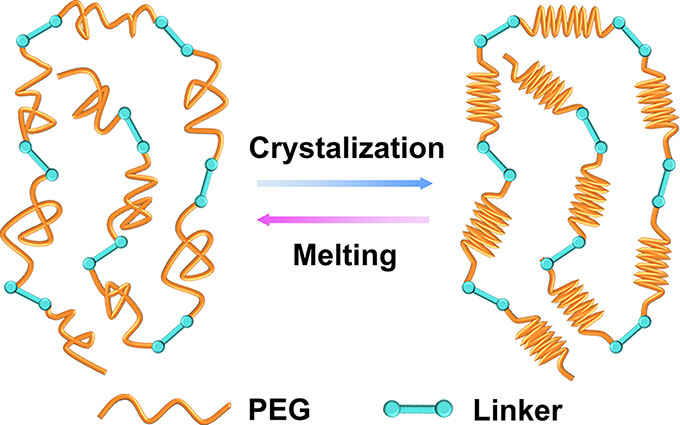
The Chinese team 3-D printed its fabric using an “ink” made from a mix of the new polymer and carbon nanotubes. As their name implies, each of those tubes was only a few billionths of a meter in length. X-ray diffraction — an imaging tool that reveals the arrangement of individual molecules — shows that the polymers change from their messy non-crystal phase at warm temperatures to an orderly crystal phase when they’re cooler. This phase changes occur between 40º and 55º Celsius (104º and 131º Fahrenheit). Changing the polymer’s chemical recipe could allow it to change phases at different temperatures.
The new fabric performed well, Zhang says — better than his team had expected. Even after being folded 2,000 times, it worked as it was designed to do.
But Granados-Focil at Clark University points out that the team never made a side-by-side comparison of the fabric’s durability with and without its phase-change component. He’d like to see such a comparison.
Why add carbon nanotubes?
The Chinese team added nanotubes to the fabric to help it conduct electricity. Nanotubes also speed up the cloth’s ability to move heat around, Zhang explains. An added benefit, he notes: The tiny tubes add “radiation resistance.”
Here, Granados-Focil explains, “They’re talking about radio waves … Wi-Fi connections, that kind of radiation.” If used to encase a smartphone, he says, others “can’t access the information.” But that trait also confuses him. Considering the fabric’s electrical conductivity, he wonders: “In what scenario would you need to combine those two?”
“But being able to absorb all that radiation and not to seem hotter or colder than the environment around you,” he notes — “that could give you some camouflage property.” This attribute might add appeal for military or defense-type applications.

Educators and Parents, Sign Up for The Cheat Sheet
Weekly updates to help you use Science News Explores in the learning environment
Thank you for signing up!
There was a problem signing you up.
Byron Jones believes ordinary fashion designers would have little need or interest in such applications. A mechanical engineer, Jones works at Kansas State University in Manhattan. But years ago, he worked with companies that actually tried developing phase-change fabrics for everyday use. “My personal opinion,” he says, “is that phase-change materials in everyday clothing is more about marketing hype than it is about useful impact.”
Here’s the problem, Jones says. Your phase-change jacket absorbs heat as you get hot, then releases it as you cool off. You hope it will keep you comfortable. But it can’t absorb all your heat. Still, absorbing even 10 percent should keep you modestly comfy. And if you walk around for about 10 minutes in that jacket, 10 percent of the heat you give off over that time would come to about 72,000 joules. (Joule is a unit of energy.) For comparison, a regular light bulb emits about 60 joules per second.

According to the team’s data, each gram of their fabric absorbs 65 joules. Not 65 joules per second. It’s 65 joules once, during the phase-change. Afterwards, the polymer stops storing heat. So to absorb 72,000 joules, Jones explains, your jacket must have 1,100 grams — or 2.4 pounds — of the phase-change fabric. And that’s like carrying around two and a half cans of condensed soup. He concludes, “No matter how you hype it, you can’t get around these physics.” So it might work for a jacket, but hardly a tee-shirt.
Jones does see some possible uses, however. It might prove useful in a situation where you only need “a few minutes” of heating or cooling capacity. “Then you go back into an environment where the phase-change material is recharged” — returned to its heat-absorbing or heat-shedding state.
Granados-Focil agrees. “This electrical conductivity business — it’s interesting,” he says. For example, in gloves, it should mean you can operate touchscreens. And it might let you touch something really hot or cold for 30 seconds, without needing clunky gloves.
This technology “isn’t going to change all of our sweaters tomorrow,” Granados-Focil concludes. But for niche-type applications, he says “it could be interesting.”
This is one in a series presenting news on technology and innovation, made possible with generous support from the Lemelson Foundation.