New spray gel moves drugs deep to treat frostbite
Nanotechnology limits damage and boosts healing in severely affected tissues

People who spend a lot of time in the frigid outdoors face a risk of frostbite — potentially devastating tissue damage. But a new gel may offer protection against that.
DieterMeyrl/E+/Getty Images
Sledding, skating, snowshoeing, skiing and more can provide hours of entertainment on short winter days. But cold temps can bring more than frosty fun. They can also put people at risk of serious — and permanent — damage to skin and other tissues. Called frostbite, such injury can be difficult to treat. But a new spray gel may provide immediate help. It can reduce frostbite damage at the same time it speeds healing.
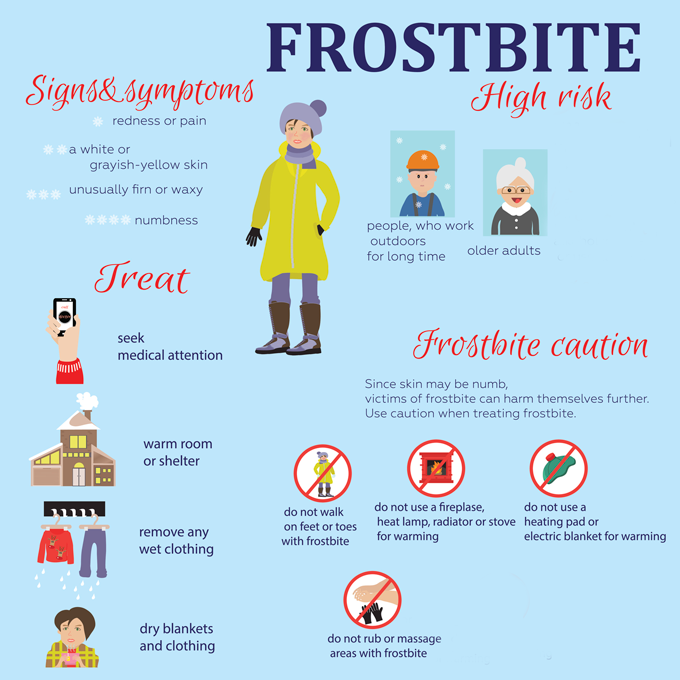
Frostbite happens when parts of the body are exposed to below-freezing temperatures. Particularly at risk are toes, fingers and ears, as well as the nose, chin and cheeks. These will receive less blood flow than will more central parts of the body. So they’re more likely to freeze. Indeed, when they get cold enough, fluids in and around the cells can crystallize. This kills those cells.
What’s more, blood flow to the extremities — areas far from the torso — will slow. That’s because the vessels bringing blood to these areas constrict. The tighter they squeeze, the less blood that gets through. It’s no accident. The body does this to retain heat in its core so that the heart and other essential organs keep working. But that reduced blood flow can starve the extremities of heat, oxygen and nutrients. Even tissues that don’t freeze can be damaged when they don’t get all the oxygen they need.
After warming up, a frostbite injury may worsen. If it turns red and swollen, inflammation may have developed. This is an important part of the immune response. But when it goes on for too long, inflammation releases cell-damaging free radicals and inflammation-promoting peptides. These can cause a different type of harm — one that makes frostbite tough to treat.
Super-small science to the rescue
To help frostbite injuries heal, a medicine would need to stop inflammation, increase blood flow and boost healing. It also would need to reach deep layers of injured tissue. A treatment that reaches only the top layers wouldn’t heal the entire wound. But getting drugs across tissues can be tricky. So Rahul Verma turned to nanotechnology.
Verma is a drug researcher at the Institute of Nano Science and Technology in Mohali. That’s in Punjab, India. He teamed up with other researchers in India to develop a gel that can be sprayed on a wound.
The gel contains two important ingredients. One is the drug ibuprofen. This common pain reliever also fights inflammation. The other drug is heparin [HEP-uh-rin]. The liver and lungs naturally make this compound. It prevents blood from clotting. Sometimes doctors even prescribe laboratory-made heparin as a blood thinner. After surgery, for instance, it can help prevent dangerous clots. (If you’ve ever had blood drawn, the collection tubes contained heparin to keep your blood liquid.) Heparin also boosts blood flow and speeds healing. That makes it an ideal treatment for frostbite.
But Verma’s team had a problem. Heparin has a negative electric charge. Being water-repellent — or hydrophobic (Hy-druh-FOH-bik) — it will not easily cross the outer membrane into a cell. Those membranes are negatively charged. (Negative charges repel each other; opposite charges attract.) To overcome this issue, the researchers packaged heparin inside super-small, membrane-walled packets. These packets are positively charged and water-loving — or hydrophilic (Hy-druh-FIL-ik). This helps the packets pass through cell membranes. They’re also tiny, just 200 nanometers (0.000008 inch) across. That itty-bitty size lets them move deeply into tissues with ease.
The scientists mixed these packets of heparin and ibuprofen with other ingredients to create a sprayable gel. Then they tested this product on rats with frostbite. Rats that received no treatment experienced only slight healing during the two weeks after their injury. Those that received heparin healed some, but not completely. Only rats spritzed with the nano-spray gel healed fully within 14 days.
Verma’s team published its findings on November 6 in ACS Biomaterials Science & Engineering.
Not yet ready for prime time?
This gel is the first step toward an important new treatment for frostbite, says Verma. Still, it will be a while before winter adventurers can buy it. “We are planning for clinical trials on humans,” he says. If these “get all positive results,” he says his center will think about releasing the spray gel for sale.
Whether some other treatment also would be needed “depends on the severity of the frostbite injury,” Verma notes. The gel should help protect the injury early on. But severe frostbite, he says, might need additional medical treatment.
The study “is a good glimpse into the kinds of background studies that are published before a new medication can be tested on humans,” says Robert M. Brickley. He works at the University of Utah in Salt Lake City. There, he’s not only an emergency physician but also a wilderness-medicine expert. Brickley’s not sure heparin would work the same on people as it did for the rats. But if it does, he says it “could be a major advance in the treatment of frostbite in remote and expedition settings.”