Scientists claim to have turned hydrogen into a metal
To do it, they squeezed gas molecules in a diamond anvil
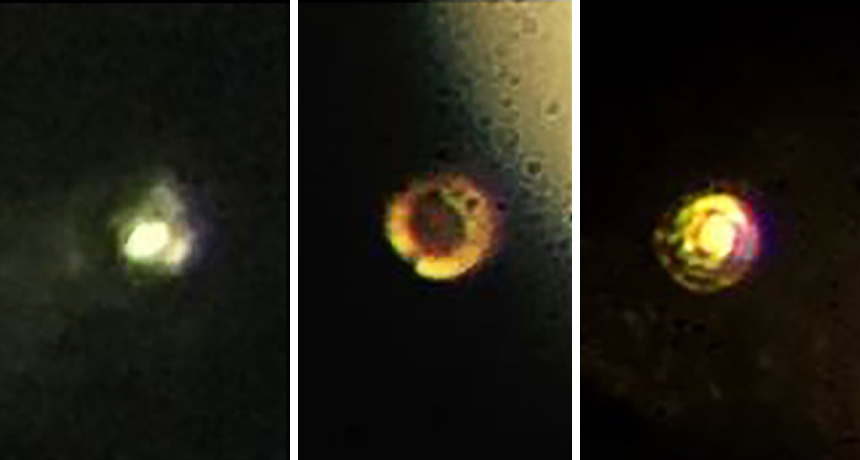
Scientists squeezed hydrogen between two diamonds. This changed it from transparent (left) to black (middle) and, finally, to a reflective state (right). That reflectivity is a sign hydrogen transformed into a metal, the researchers claim.
Ranga Dias, I.F. Silvera
Scientists may have just given hydrogen a squeeze strong enough to turn it into a metal. The important point here is that they “may” have. In fact, some critics strongly dispute the new claim.
Under extremely high pressures hydrogen may become reflective. Researchers at Harvard University in Cambridge, Mass, have just claimed to have seen it. That reflectivity is a key trait of metals. Their feat required compressing hydrogen to 4.9 million times atmospheric pressure.
Isaac Silvera and Ranga Dias reported their findings online January 26 in Science.
If correct, this crushing work would end a decades-long search for a material that could have unusual properties, such as superconductivity. (That’s the ability to conduct electricity without resistance.)
Most superconductors work only at extremely cold temperatures. But some scientists have calculated that metallic hydrogen might prove a relatively high-temperature superconductor. It might have this trait even at room temperature — higher than any other known superconductor. If so, the new discovery would raise hopes that superconducting metallic hydrogen could be used in power lines. That could make transmission of electricity vastly more efficient.
“I think there’s a good chance that it’s correct,” David Ceperley says of the new claim. A theoretical physicist, he works at the University of Illinois at Urbana-Champaign. The pressure at which the hydrogen became reflective is about where theoretical physicists have calculated that a metal should form, he says.
What the new study did
To put the pressure on hydrogen, scientists capture it as a gas between the tips of two diamonds. Then they squeeze tips of that diamond “anvil” together. It’s not easy. “The problem in making metallic hydrogen has been that the predicted pressures have been very high,” says Silvera. “Diamonds always break before you can obtain those pressures.”
To prevent that, his team smoothed the surface of the diamond. This got rid of any defects. The scientists also covered the gems in a thin layer of aluminum oxide. That layer kept the hydrogen from diffusing inside the diamond and triggering cracks.
The researchers also cooled their lab setup to no more than 83 kelvins (−190° Celsius or -310° Fahrenheit). As the scientists ratcheted up the pressure, the hydrogen first turned black. That indicated a possible semiconducting phase. Eventually the hydrogen turned reflective. That pointed to it having become a metal. Metallic hydrogen could be either a solid or a liquid, Silvera notes.
Such experiments are tricky. Indeed, only a few research teams in the world can perform them. One risk: The hydrogen may leak from the chamber without the scientists realizing it. However, Silvera says, “We’re sure we have hydrogen in there.”
Some earlier attempts to make metallic hydrogen have monitored the element as the pressure was ramped up. The goal was to study the material’s transition — and to make sure the hydrogen did not escape along the way. To do this, scientists use a technique called Raman spectroscopy (RAH-mun Spek-TROS-koh-pee). It involves shining a laser through the diamonds, then observing how the light scattered. But at pressures this high, lasers could cause the diamonds to break, Silvera says. So the researchers used lasers only after the sample had reached the metallic state.
Silvera’s group is not the first to announce the discovery of metallic hydrogen. But earlier claims of finding the metal have been overturned. Even in this case, “It’s not the last word,” says Ceperley. However, he adds, “It should encourage all the other groups to come out and try to reproduce it.”
But not everyone is buying that the Harvard group achieved what it’s claiming it did. Among them is Eugene Gregoryanz at the University of Edinburgh in Scotland. This physicist works on similar experiments. And he claims that the new paper should never have been published. That it was, he argues, represents a failure of the journal’s review process.
Based on what is reported in the new paper, he even doubts that the claimed pressures were reached. He points out that the researchers gave data from only one experiment. “How is it possible to do only one experiment and claim such a big thing?” he asks.
Physicist Alexander Goncharov works at the Carnegie Institution for Science in Washington, D.C. He, too, challenges the Harvard team’s conclusions. “It’s not shown whether they have hydrogen at all at high pressure,” he says.
Clearly, this story isn’t over.