New ultrasound treatment kills off cancer cells
It spares healthy cells while taking out cancerous ones
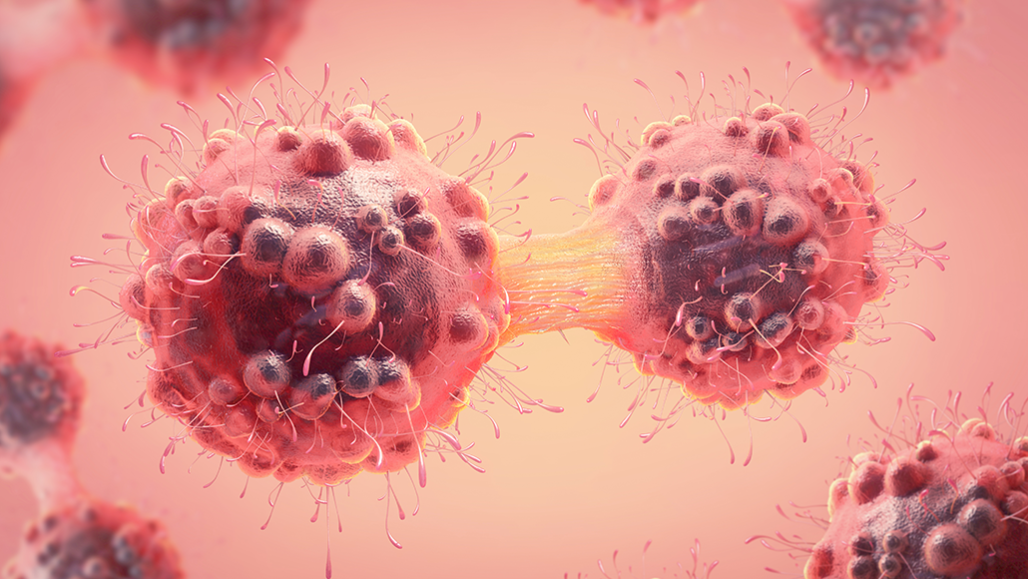
Cancer cells divide over and over, allowing them to grow rapidly — and spread. A new ultrasound treatment targets its cell-killing energy on these cells, sparing healthy ones.
Christoph Burgstedt/iStock/Getty Images Plus
Most cancer treatments involve surgery, chemical poisons or toxic radiation. Because they tend to take out healthy cells along with cancerous ones, these treatments can leave patients tired, hurting and more. So researchers are looking for new approaches that spare the healthy cells. One new idea would destroy cancer cells with ultrasound energy. Even this treatment, however, can sometimes damage healthy tissue. But a new development may help. It limits the ultrasound energy’s damage to only the cancer cells. Healthy cells should suffer little if any harm from it.
It’s exciting, says David Mittelstein of his team’s findings. Mittelstein is a biomedical engineer at the California Institute of Technology, in Pasadena. Low-intensity ultrasound, he says, “may allow physicians to target cancer cells based on their unique physical and structural properties.” Any spillover of the energy should cause little harm to healthy tissue.
The treatment sends out pulses of sound waves — energy — that have a frequency above 20,000 hertz (cycles per second). That’s too high for our ears to hear. (That’s also what makes it “ultra” sound.) Medical imaging relies on very short pulses of this low-intensity ultrasound.
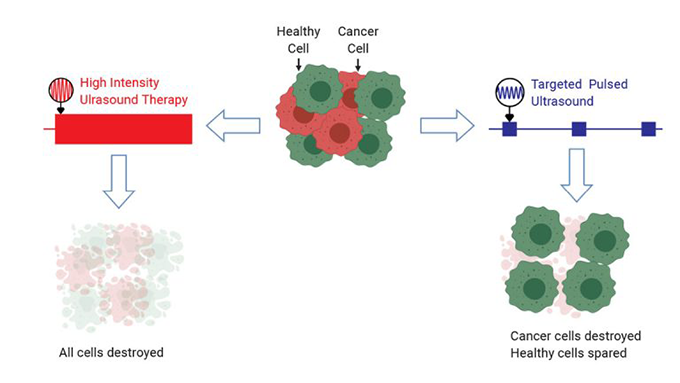
Doctors had already used high-intensity ultrasound to kill cancer cells. These sound waves send lots of energy to a small, focused area. The waves vibrate water inside cells within that area. This causes the cells to heat up. A lot. Targeted cells and their neighbors can reach 65° Celsius (149° Fahrenheit) in just 20 seconds. This kills cancer cells. The down side: It kills healthy ones, too.
Mittelstein’s team wanted to try something different.
Another Caltech lab had studied effects of low-intensity ultrasound on cancer cells. These cells differ from healthy ones. They have a bigger nucleus. They’re softer, too. This other Caltech team created computer models of cancer cells. These models suggested that low-intensity ultrasound might kill those cells. The process, Mittelstein explains, is “similar to how a trained singer can shatter a wine glass by singing a specific note.”
This idea hadn’t been tested, however. So his team set out to do that.
First, they mixed cancer cells with healthy blood cells and immune cells. The cells were all suspended in a liquid. Then the scientists directed short pulses of low-intensity ultrasound at this suspension.
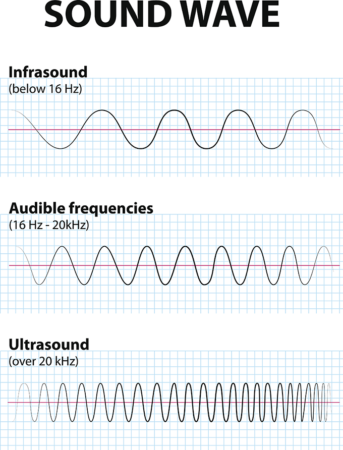
The team tested different ultrasound frequencies (ranging from 300,000 to 650,000 hertz). They also tested different pulse durations (from 2 to 40 milliseconds). One minute of 500,000 hertz ultrasound, delivered in 20-millisecond bursts, killed nearly every cancer cell. It didn’t hurt the blood cells. It also left more than eight in every 10 immune cells unharmed. Mittelstein rates it a huge success.
A role for microbubbles
The treatment caused super-small microbubbles — likely tiny bubbles of air present in the fluid — to merge. The ultrasound waves caused these bigger bubbles to oscillate (move back and forth). The oscillation caused these microbubbles to grow, then violently collapse. To kill cancer cells, Mittlestein reports, “microbubble oscillation was necessary — but not sufficient.” Microbubbles oscillated in both healthy and cancer cells. “But only the cancer cells,” he notes, “were vulnerable to certain frequencies of ultrasound.”
More damage occurred when the ultrasound waves bounced back to hit the cancer cells more than once.
The initial ultrasound waves are known as traveling waves. They move out from the machine that produces them. But when those waves hit a surface of some type, they can reflect back — into the oncoming traveling waves. The colliding waves combine to form a special pattern known as “a standing wave,” Mittelstein notes. And this wave has some “special stationary spots called ‘nodes,’” he explains. At these, the pressure remains constant. Some other stationary spots, called “anti-nodes,” also develop. In them, he says, “the pressure goes up and down at twice the amplitude [height] of the traveling wave.” In the end, bubbles in the standing wave oscillate more than do those in a normal wave. And that extra oscillation proved essential to killing cancer cells.
The team suspects the standing wave brings microbubbles closer together. That then boosts the ultrasound energy deposited on the cells, Mittelstein says. Not all cells respond equally to this standing wave. Which do will depend on their physical properties. Here, only cancer cells were harmed.
In his experiment, Mittelstein used a reflector to bounce the sound waves back into the suspension to create that standing wave. Bouncing ultrasound against bone might provide the same type of boosted impact, he suspects.
The team published its findings January 7 in Applied Physics Letters.
This study is exciting, says Timothy Meakem. He was not involved with the study. He does, however, know about ultrasound’s value in medicine. He works at Focused Ultrasound Foundation in Charlottesville, Va., as its chief medical officer. If the effect seen in these cells also occurs in people, he says, it would let doctors target cancer cells in ways not currently possible.
However, he cautions, this technique is not ready for use in patients. This is just the first step in the process of developing a new treatment. But if the next stages go well, it “might be a huge benefit to patients.”
Mittelstein is already moving ahead. His team’s next experiments will go beyond targeting cells in a liquid. They will focus on mounds of cells, which model a cancerous tumor. If they get similar cell killing in treated tumors, he says, “we think this therapy could make a significant impact in cancer therapy.”
This is one in a series presenting news on technology and innovation, made possible with generous support from the Lemelson Foundation.