Nature shows how dragons might breathe fire
Reliably bringing all of the ingredients together without harming the dragon could, however, get explosive

Dragon fire is the realm of fantasy. But if a dragon did exist, nature’s got the tools to give it fire-breathing abilities.
Grandfailure/iStockphoto
No fantasy world is complete without a fire-breathing dragon. But if dragons were real, how might they get that fiery breath? Nature, it seems, has all the parts a dragon needs to set the world on fire. The creatures just require a few chemicals, some microbes — and maybe tips from a tiny desert fish.
Fire has three basic needs: something to ignite the blaze, fuel to keep it burning and oxygen, which interacts with the fuel as it burns. That last ingredient is the easiest to find. Oxygen makes up 21 percent of Earth’s atmosphere. The bigger challenges are sparking and fueling the flame.
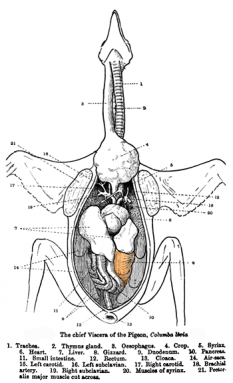
All it takes to strike a spark is flint and steel, notes Frank van Breukelen. He’s biologist at the University of Nevada, Las Vegas. If a dragon had an organ like a bird’s gizzard, it could store swallowed rocks. In birds, those rocks help break down tough foods. Swallowed flint might rub against some steel inside the dragon, sparking a flame. “Maybe what you have is sort of scales that are flint-like and click together,” van Breukelen says. If the spark was close enough to a very sensitive fuel, that might be enough to ignite it.
But some chemicals don’t need that initial spark. Pyrophoric molecules burst into flame the instant they contact air. Consider the element iridium, says Raychelle Burks. She is a chemist in Texas at St. Edwards University in Austin. Iridium burns different colors when it becomes part of various molecules. One of them burns a warm orange or red. Another burns a violet-blue. (That’s one way to get the blue flame of the zombie ice dragon in George R.R. Martin’s Game of Thrones series.)
Unfortunately, iridium isn’t common, especially in biology. “There are a lot of cool elements on the periodic table, but [living things] only use a few,” Burks explains.
There are other pyrophoric chemicals that a dragon might find a little closer to home, notes Matthew Hartings. He’s a chemist at American University in Washington, D.C. Assume that dragons like caves, he begins. “If you’re living amongst a bunch of rocks, you’ll have access to a high amount of iron.”
Iron can react with another chemical, hydrogen sulfide. This is a flammable gas that smells like rotten eggs. It is found in crude oil. When hydrogen sulfide and iron get together — in a rusty oil pipe, for example — the result is iron sulfide. Combine it with air and you’ve got an explosive mix. Iron sulfide is sometimes the culprit when gas pipelines or tanks blow up.
Another explosive option comes from Anne McCaffrey’s series The Dragonriders of Pern. McCaffrey describes her dragons chewing on rocks containing phosphine — a chemical made of one phosphorus atom and three hydrogen atoms. In gas form, phosphine is very flammable and explodes on contact with oxygen. It’s also very toxic: Just seven drops of its liquid form can kill someone.
Burning burps
Fictional dragons often spout flaming gas. But a gas would present problems, Hartings says. Gas, he notes, expands to fill available space. To keep it contained, a dragon would have to keep that gas under pressure.
Chemicals like phosphine, therefore, aren’t the perfect dragon-fire solution, Hartings says. The boiling point for phosphine is -84° Celsius (-120° Fahrenheit). At room (or dragon breath) temperature, it’s a gas. “You’d have to really compress it,” he says, to make it a liquid that a dragon could store and use.
Also, Hartings notes, gases are difficult to control. If a dragon blew some fiery gas into the wind, the flames might wash back on the creature and singe its face. “You have a much better chance of controlling your flame spray if you’re pushing liquid rather than a gas,” he explains.
A liquid also would help a dragon avoid burning itself, Hartings notes. The liquid with its flammable gas would ignite as soon as it hit air. Speed is key. “As long as you are shooting it out fast enough, [the] particles don’t hit the air until they are far enough away from your face,” he notes.
A combination of liquid and gas might work even better, Burks suggests. In an aerosol spray, tiny liquid droplets are suspended in a pressurized gas, which spurts out when it is released. If a dragon were to shoot an aerosol spray, it could look like a gas, with some of the properties of a liquid. “In a fine aerosol spray, it would look like the dragon is spraying fire,” Burks notes. The aerosol would spread out, she says, “and the minute it hits air — kaboom!”
Something fiery, something fishy
Plenty of liquids in nature will burn. Living things already produce two of these that might work for a dragon: ethanol and methanol. Both are alcohols often burned as fuels.

“Certainly, we know that yeast makes ethanol,” Hartings says. These single-celled fungi transform sugars into alcohol. That’s why they’re used to brew beer and make other alcoholic beverages. A dragon with a bellyful of yeast is not as silly as it might appear. Yeast are part of the microbial community that lives on and in people and other animals.
Methanol first requires methane. Ruminants — including cows, goats, giraffes and deer — make methane during digestion. Certain bacteria can turn methane into methanol, Hartings notes. A dragon that got enough fiber in its diet to make methane could pass that gas on to its bacterial buddies, which would convert it into methanol.
But those bacterial coworkers might not even be needed. The Devil’s Hole pupfish doesn’t bother with them. It is a tiny, incredibly rare species found in Devil’s Hole — a single naturally heated pool in Nevada. This fish can whip up its own whisky in a pinch, van Breukelen and his colleagues have shown.
Temperatures in Devil’s Hole reach 33 °C (91 °F). There is very little oxygen in the water to start with. When it gets hot, the oxygen levels drop even lower — too low for the fish to breathe. So pupfish stop using oxygen. Instead, they produce energy anaerobically — without oxygen. In the process, their bodies make ethanol.
The fish produce 7.3 times more ethanol than fish living in cooler water, notes van Bruekelen. He and his colleagues published their fishy findings in 2015 in the Journal of Experimental Biology.
A dragon might be able to produce ethanol under similar circumstances. However, van Breukelen says, it’s not quite so simple. “I don’t think there’s a way to keep ethanol. I don’t think you could store it,” he says. The reason: It seeps through everything. Ethanol, he explains “goes right through membranes.” Those include the membranes that surround cells and organs. When pupfish produce ethanol, the chemical ends up throughout the fish. It would not pool as a concentrate in some pouch or organ. So any dragon that made ethanol would have trouble storing enough to get a decent flame going.
The pupfish won’t be setting the world on fire — nor will dragons. One is a tiny fish, and the other isn’t real. Both, however, offer an excuse to use our imaginations to apply science to the fantastic.
Technically Fiction is a blog that finds the science in the realm of the fantastic. Have a comment or a suggestion for a future post? Send an email to sns@sciencenews.org.