
Physics
Explainer: What are the different states of matter?
Most people know solids, liquids and gases — but what about the four other states of matter?
Come explore with us!

Most people know solids, liquids and gases — but what about the four other states of matter?
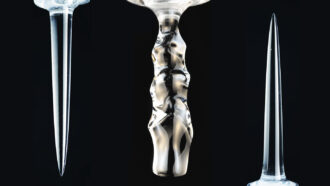
The newfound — and at times quirky — shapes reflect the density of water surrounding submerged ice.

A fat molecule's three long chains of carbon and hydrogen atoms repel water, stash energy and keep living things warm — even in the bitter cold.

Baking soda volcanoes are a fun demonstration, and with a few tweaks they can be an experiment, too

New measurements of a weird but simple atom, one without a nucleus, suggest it may have unexpected properties. Scientists find this troubling.

Pour out liquid water into a solid ice tower. We outline the conditions you’ll need to turn this demonstration into a super-cool experiment.

Snot oozed by a marine tube worm can glow for up to 3 full days. The secret of how this works might lead to long-lasting lights that glow on and on.

Feared equipment shortages due to the COVID-19 pandemic have prompted research teams to develop novel technologies to help oxygen-starved lungs.

Making rock candy at home takes a lot more sugar than you might think. Why? This experiment will show you why.

Food scientists now show that adding these tiny plant particles to ice cream may delay the rate at which this treat melts into a soupy mess.