Explainer: What is a clinical trial?
Countless numbers of people volunteer for research studies to help answer medical and health questions

Volunteers who take part in clinical trials usually start by answering a host of questions about their health and habits. Those who qualify to take part may be asked to take experimental drugs, experimental therapies (such as acupuncture to treat headaches), new food supplements or exercise regimens.
nensuria/iStockphoto
Let’s say your doctor prescribes some medicine. Perhaps it’s a pill. If you live in the United States, Europe or many other parts of the world, that medicine will have undergone tests. Before that medicine makes it into your hand, researchers will have ensured that it works well and is safe.
And they did this through clinical trials.
Such research trials always involve human volunteers. The people can be any age, from babies to the elderly. It all depends on who might benefit from a new treatment or behavioral change. Scientists refer to what is being tested in these studies as an intervention.
A clinical trial generally divides its volunteers into two or more groups. As its name implies, one group — the “intervention group” — always receives the intervention. Other groups may receive a different amount or type of treatment. Yet another group may receive no treatment at all. These other comparison groups are known as controls. Researchers compare what happens to the people in the intervention group against what happens to the controls.
New medicines or vaccines are one type of intervention. But there are many others. Talk therapies, such as what a psychiatrist might use, are another type. So are diets, exercise regimens and the educational videos that may be shown to teens.
Or researchers may want to assess how well a medical device works. For instance, will some new “pen” that injects the drug epinephrine (Eh-pih-NEF-rin) halt a potentially life-threatening allergic reaction fast enough? Medical acupuncture trials may test at which sites on the body the needles should be inserted.
Each new clinical trial builds on existing knowledge. This can come from other clinical trials, from studies of people that don’t involve interventions and from research in animals. It might even build on research in cells or tissues in the lab, or on computer analyses of the shape and properties of new molecules.
For new drugs or vaccines, the early work begins in a laboratory. That’s where the ingredients all come together. When researchers want to understand how something will function in people, they may first use animals as a stand-in. Why animals? One reason is that they tend to be shorter-lived. Probing the life-long effects of some treatment may take only days or weeks in short-lived insects. To study the same thing in rodents may take only a few years. To assess the same impacts in people, however, could take decades.
Those early steps can uncover a lot about how a treatment might work, or whether it might cause unwanted effects. Data from these trials also may suggest how much or how often a treatment might need to be used. And researchers can use such data to help figure out who might benefit most. Only then will they be ready to start testing the intervention in people.
How a clinical trial works
Consider a recent clinical trial that looked at depression and insomnia in youth.
Depression is a mood disorder. Its victims can feel persistent or recurring bouts of sadness. Some may feel hopeless. Very often, depressed people may have trouble falling asleep. If they awaken in the middle of the night, they may be unable to get back to sleep.
Greg Clarke wanted to see if it was possible to reduce symptoms of depression by treating someone’s sleep problems — insomnia — as well as the depression. As a clinical psychologist at the Kaiser Permanente Center for Health Research in Portland, Ore., Clarke studies depression, insomnia and anxiety.

Earlier clinical trials had shown promising results in adults. Now Clarke and his colleagues wanted to know if the sleep therapy would help younger patients, too.
They recruited 41 people, all 12 to 20 years old. Each was assigned to a group at random. (Usually this is done by a computer program.) Such “randomization” is an important step. It helps reduce differences between the groups. Without randomization, all of the people most likely to benefit from treatment could end up in the intervention group. That could make the treatment appear more effective than it really is.
“When you finally finish the study,” Clarke says, “you should really have only one thing different between the two groups.” And that would be “the treatment that they were exposed to.”
All recruits in Clarke’s trial received therapy for depression. For the intervention, some of these people also received therapy for insomnia. Not the control group. They just received instructions on good sleep habits.
By the end of the study, people in the intervention group slept, on average, 40 minutes longer than did those in the control group. A higher percentage of people in the intervention group also recovered from depression — and they recovered faster.
Many clinical trials also are “blinded.” This means that the people involved in the trial do not know which of them are receiving the intervention — and which are not. By being “blind” to these details, people cannot influence the outcome of the trial. When the participants don’t know which group they are in (intervention or control), the trial is known as single-blinded. In a double-blinded study, both the participants and the researchers are kept in the dark — until the entire study is over.
The information is collected in stages
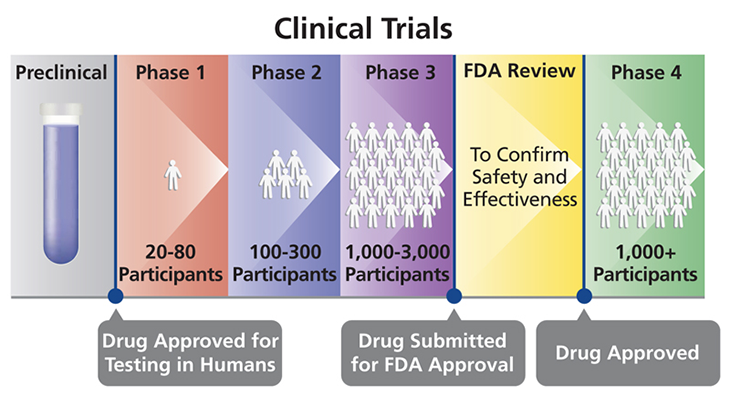
Research on drugs or treatments can take place in four phases, designated by the Roman numerals I to IV. Each phase is a separate type of clinical trial.
Phase I trials are small. They typically include only 20 to 80 people. These trials test the safety of a treatment, usually in healthy people. They also help to figure out the right dose of a drug or therapy. According to the U.S. Food and Drug Administration, roughly seven in every 10 drugs move on from a phase I to a phase II clinical trial.
Phase II trials enroll 100 to 300 people. Each recruit will have the illness or condition that might be helped by the treatment. These trials test how well the treatment works to help that condition. They also try to identify all side effects (nearly every treatment has some). Such trials can last for months to a few years. Roughly one in every three treatments tested this way will move on to the next type of trial.
Phase III trials scale up, recruiting some 300 to 3000 volunteers, all having the target condition. The larger numbers of volunteers provide more information. These trials compare the new treatment against existing treatments for the same condition to see which works better. These trials also continue to look for side effects. Phase III trials follow people for one to four years. Data from this stage might be used to get a new drug or treatment approved for widespread use in people with the condition. Only about one in every three or four drugs does well enough to move on to the final type of clinical trial.
Once a new drug or medical device is approved, medical professionals can prescribe it to the general public. But researchers often will continue to track it in what’s known as a phase IV trial. Here, researchers again want to confirm that the intervention is working. More importantly, they want to check that the long-term benefits greatly outweigh any side effects. These trials can follow thousands of treated people.
Story continues below image.
Probing benefits of vitamins and more
The vast majority of clinical trials study the impacts of drugs and other therapies. But even the potential benefits of foods and nutrients might be studied in a clinical trial.
That’s what JoAnn Manson at Harvard Medical School in Boston, Mass., and her colleagues are doing. They are directing a long-running trial known as VITAL. (Its name is an acronym for VITamin D and OmegA-3 TriaL.) Vitamin D is a nutrient that the body converts into a hormone to boost bone and muscle health. Omega-3 fatty acids are the heart-healthy fats in fish oil and some plants. This massive study, begun in 2010, has been following almost 26,000 U.S. men and women. Its main goal: to tease out whether high doses of vitamin D and omega-3 fatty acids can prevent cancer, heart disease or stroke. This study will be on the lookout for signs of other benefits as well.
Data collected so far have led to a flurry of research papers. So far, Manson and her colleagues have been tracking the health of all of their recruits. These data will define the baseline, or starting point, of the volunteers’ health. Then the team can see whether people who get these daily food supplements end up healthier than people who don’t.
“A single study,” Clarke notes, “is not enough to draw a conclusion.” In fact, “sometimes even a dozen studies may not be enough” — especially if they did not include enough people. What’s more, he adds, it’s important that related studies all ask the same or similar questions. But when they do, the data they acquire should paint a picture of how valuable a new intervention can be.
JoAnn Manson/Harvard University







