Analyze This: Real data on lead levels in school drinking water
Monitoring for lead (as in these data) is an important part of identifying risks to health

Water that appears clear and clean can be unsafe if it contains high amounts of lead, a toxic metal.
Joseph Thomas Photography/iStockphoto
You turn on the faucet to get a glass of water. The liquid comes out clear and odorless. It’s safe, right? Maybe not. In some places, such as Flint, Mich., water coming out of the tap may contain lead, a toxic heavy metal. Finding it can be hard because lead-tainted water has no unusual color, odor or taste. But once inside the body, that lead can damage nerves — including those in your brain!
Drinking a single glass of lead-tainted water won’t cause acute poisoning. The problem is that once lead gets into the body, it doesn’t want to leave. So over time, small exposures can add up. And because lead moves into the bones, it can stay there for years — to later enter the bloodstream and flow throughout the body — again and again.
Babies and young children are especially vulnerable to lead’s poisoning. Symptoms range from headaches and stomachaches to learning disabilities. Severe problems include slowed or stunted bone and muscle growth, and damage to the kidneys. Lead can even permanently reduce a child’s IQ.
There are many ways that lead can enter drinking water. The most common way is from the pipes that connect a home or school to a town’s main water-supply lines. In many cities, there are still lots of old pipes made of lead. Lead also can be found in the material used to seal connections between pipes. (That sealant has been banned but may still exist in some pipes.) The metal in many faucets, fountains and water spigots also may contain lead.
Whether that lead ends up in your drinking glass tends to depend on how acidic the water is. Acidity is measured by a pH scale. Any water with a pH less than 7 is acidic (and the lower the pH the more acidic it is). When drinking water is acidic and contains a lot of calcium carbonate, it becomes corrosive. That means it breaks down metal.
So when corrosive water sits in pipes overnight, it can leach out a lot of any lead that’s there. This is why it’s a good idea to run cold water for one to two minutes first thing in the morning or after a vacation. That will get rid of the water that was collecting any lead over the past 8 or more hours.
The best way to avoid lead poisoning is to avoid exposure. While lead in water can’t be seen or smelled, there are tests to detect it. If those tests reveal that the water has a lead level higher than 15 parts per billion (ppb), the U.S. Environmental Protection Agency (EPA) recommends that people take action to reduce the contaminant. But even low levels are not safe — just safer.
If you are unsure of the safety of the water you’re drinking, there are a few things you can do. If your home gets its water from a public water system, there may be a Consumer Confidence Report that will include information about lead. Ask your parents if they received such a report. Or search for the report in the EPA’s database online. Some water-supply companies will even come to your home and do a free lead test.
People also can themselves test the water coming out of the faucets. This is a good option where there is no Consumer Confidence Report for your area or where you get drinking water from a well. Testing the water in your home is a good idea if you live in an older home, because it is more likely to have lead in pipes or faucets. If you’re using a store-bought lead-test kit, make sure to send your samples to an EPA-certified laboratory. They use methods to test water that are approved by the EPA.
Even when those tests come out negative, you may want to consider using only filtered cold water for cooking and drinking. Any water filter used to reduce lead should be certified by NSF International or the Water Quality Association. (The EPA doesn’t test water filters.) There are several kinds of filters available, but the carafe style filters are often the least costly. Make sure to change the filter regularly to ensure that it keeps your water clean.
School districts around the country regularly test their water for lead. Last year, for instance, Portland Public Schools in Oregon had all of their buildings tested. Preliminary data from these tests are now available online. Some of the data from three schools are graphed below.
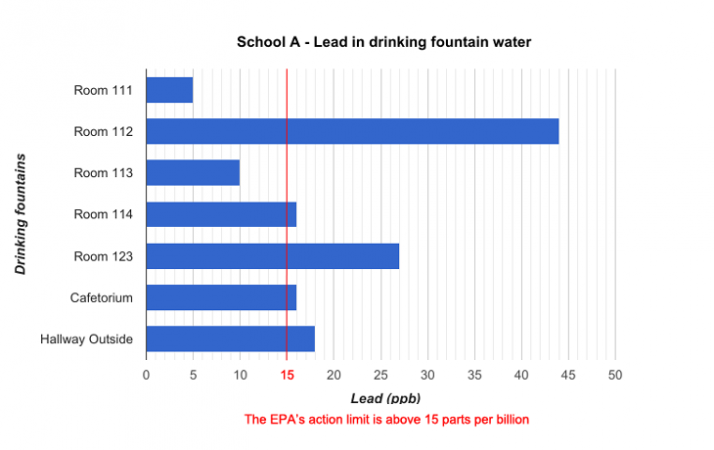
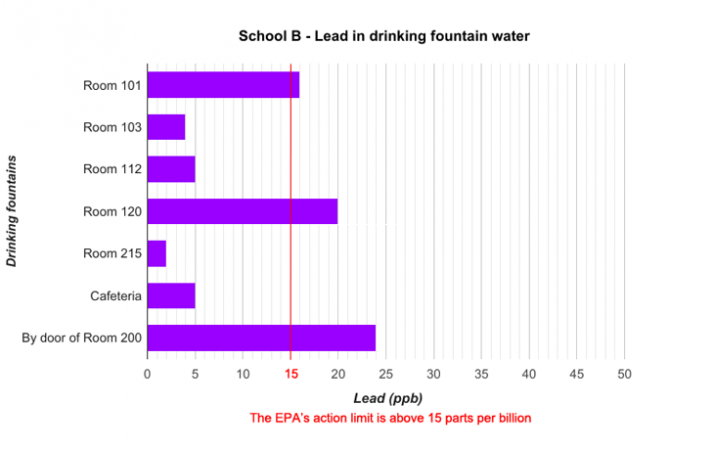
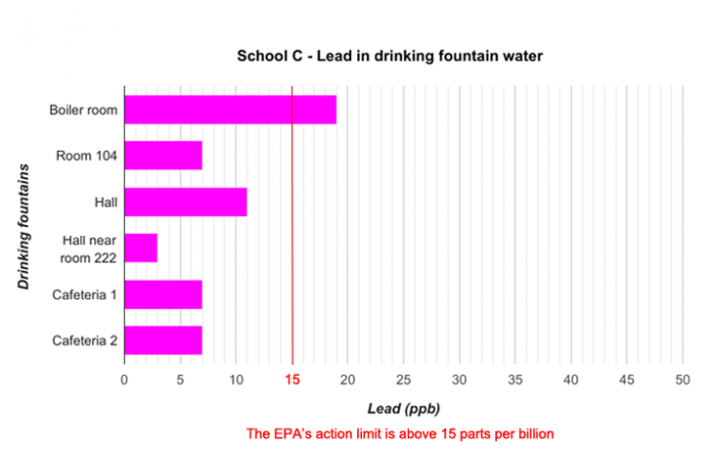
Data dive:
The data graphed include six or seven locations from each of three Portland schools. The school district tested nearly 100 buildings. Are there enough data here to make any conclusions about the water in Portland schools? What about for schools A, B and C?
Each school had its water tested by a different lab. Do you think this might have affected the results of the tests? Explain your reasoning.
Based on the data presented in the graphs, how do you think each school should respond to the results of the testing? Should students drink the water in the school? Explain your reasoning.
If the water was tested again at each water fountain, might the results be different? Explain your answer.
How else might you present these data?
Analyze This! explores science through data, graphs, visualizations and more. Have a comment or a suggestion for a future post? Send an email to sns@sciencenews.org.







