Scientists Say: Surface tension
This effect is what allows some bugs to walk on water
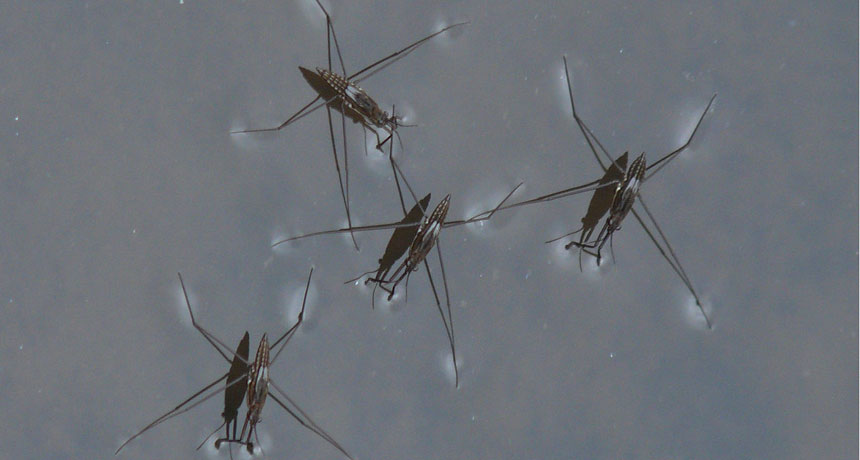
Surface tension creates just enough cohesion to hold up tiny bugs — like these water striders.
CORY/WIKIMEDIA COMMON (CC BY-SA 2.1 JP)
This effect is what allows some bugs to walk on water

Surface tension creates just enough cohesion to hold up tiny bugs — like these water striders.
CORY/WIKIMEDIA COMMON (CC BY-SA 2.1 JP)