2020 chemistry Nobel goes for CRISPR, the gene-editing tool
This ‘molecular scissors’ technology was developed just eight years ago
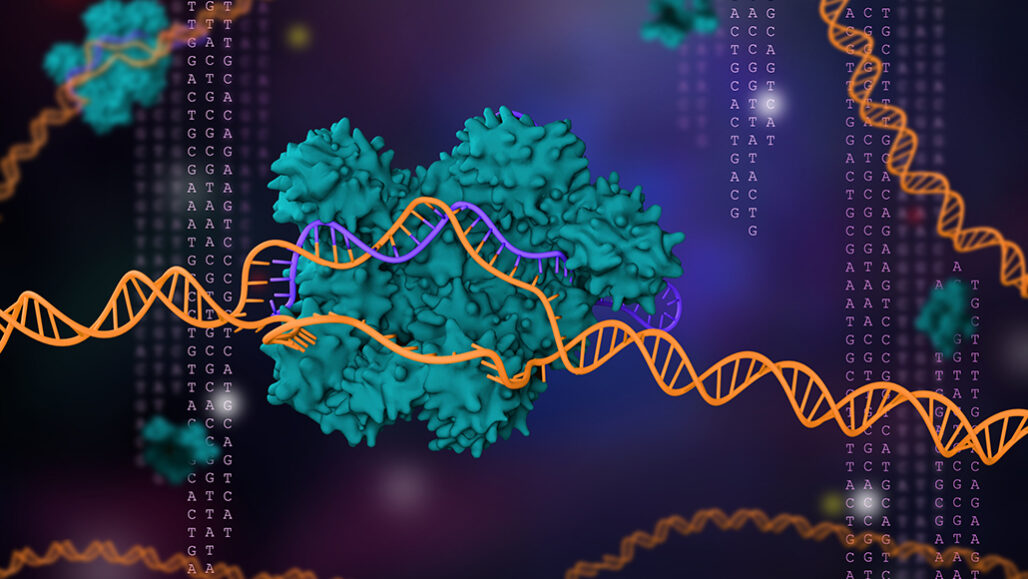
Emmanuelle Charpentier and Jennifer Doudna devised a powerful gene-editing tool called CRISPR/Cas9 (illustrated). In less than a decade, it has revolutionized molecular genetics and gene therapy. It also netted the scientists the 2020 Nobel Prize in chemistry.
Meletios Verras/iStock/Getty Images Plus
Jennifer Doudna and Emmanuelle Charpentier helped turn a bacterial defense into one of the most powerful tools in genetics. For their efforts, they have just received the 2020 Nobel Prize in chemistry.
“The genetic scissors were discovered just eight years ago,” points out Pernilla Wittung-Stafshede. She’s a member of the Nobel Committee for Chemistry. Already, she notes, these “fantastic” scissors can cut DNA where you want. In doing so, they have “revolutionized the life sciences,” she says. “We can now easily edit genomes as desired — something that before was hard, or even impossible.”
Wittung-Stafshede spoke at an October 7 news conference in Stockholm by the Royal Swedish Academy of Sciences. The prize was announced at this event. “Only imagination sets the limits for what this chemical tool … can be used for in the future,” she said. “Perhaps the dream of curing genetic diseases will come true.” She cautions, however, that ethics and law should be used to decide where and how such a powerful tool should be used.
The win was “extremely highly anticipated,” adds Luis Echegoyen. He’s a chemist at the University of Texas at El Paso and president of the American Chemical Society. Even though time from discovery to Nobel Prize is typically much longer, this award for CRISPR is “long overdue,” he says.
Doudna and Charpentier will split the 10 million Swedish kronor prize, about $1.1 million.

Learning from microbes
Bacteria and archaea have used CRISPR/Cas9 to fight viruses for millions — perhaps billions — of years.
CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats. In essence, these short, repeating bits of DNA sandwich bacteria’s version of the FBI’s most wanted list: invading viruses. Every time bacteria encounter a virus, they take a DNA mug shot of it and file it in between the repeats. The next time the bacteria encounter that virus, they make RNA copies of the mug shots. Those RNA photocopies then team up with another bit of RNA. It’s known as a trans-activating CRISPR RNA, or tracrRNA. And it sends out an all-points bulletin known as a guide RNA. Guide RNAs then ferry a DNA-cutting enzyme — Cas9 — to the virus. There the enzyme chops and eliminates the threat.
Molecular scissors
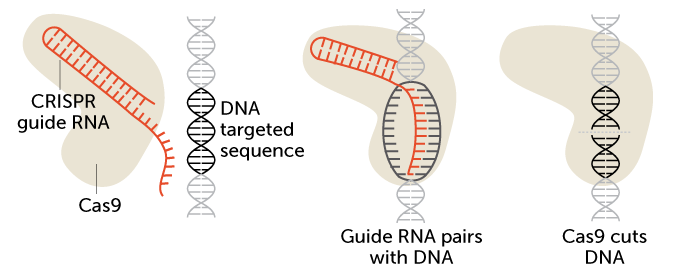
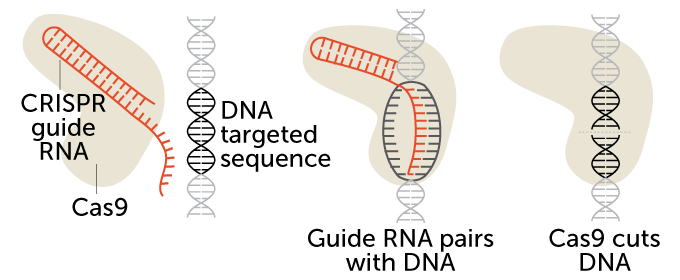
CRISPR/Cas9 is a two-part gene-editing tool. The first part is made up of a genetic guide made of RNA. The second part is an enzyme, Cas 9, that cuts DNA. The guide RNA brings the enzyme to a particular spot in an organism’s DNA that researchers wish to cut (see the targeted sequence here). That spot is a chemical match for the RNA. Once moved to the right spot, Cas9 snips the DNA.
How CRISPR/Cas9 works
Doudna works at the University of California, Berkeley. Today, Charpentier directs the Max Planck Institute for Infection Biology in Berlin, Germany. The two met in 2011 at a conference in Puerto Rico. “We walked around Old San Juan and talked about CRISPR/Cas9,” Doudna recalled during a virtual news conference on October 7. The two decided to team up to study the bacterial defense system. They ended up turning it into a gene editor.
Their innovation was to fuse the mug shot RNA to the tracrRNA. This created a single guide RNA. And the researchers realized that the mugshots didn’t have to be molecular pictures of viruses. Instead, by replacing the mug shot with RNA that matches a gene, the scientists could direct Cas9 to snip that gene — or any gene, really.

Educators and Parents, Sign Up for The Cheat Sheet
Weekly updates to help you use Science News Explores in the learning environment
Thank you for signing up!
There was a problem signing you up.
Its importance was clear from the beginning
The major paper that Doudna and Charpentier published on this has been cited more than 9,500 times. That’s almost once every eight hours since it first came out in 2012, says David Liu. He’s a chemical biologist and Howard Hughes Medical Institute investigator at Harvard University in Cambridge, Mass. (He and others have altered the original CRISPR system so that researchers can use it in a range of new ways.)
CRISPR’s promise was immediately apparent, says Stanley Qi. He’s now a bioengineer and biotechnologist at Stanford University in California. But he had been a student in Doudna’s lab. There, he had a ringside seat to the discovery. And Qi says he knew back then that CRISPR would do great things. “In these eight years there have been so many breakthroughs and advances,” he says, “it’s far beyond my expectations.”
Doudna and Charpentier “have continued to look at the broad category of CRISPR” enzymes, Qi says. Their ongoing work has contributed new insights on how this system evolved and works in nature. Doudna has done work to show what the Cas9 looks like and how it works. Her work helped other scientists make CRISPR more accurate, he says.
Many researchers have now taken these genetic scissors to the next step, using CRISPR/Cas9 to cut and edit genes in human cells. Scientists rave about how cheap and easy it is to use CRISPR. With it, researchers have edited genes in a wide variety of animals, including dogs, mice, snails, cows and mosquitoes.
The tool also has been used to encode data and store movies in bacterial DNA. Plants and mushrooms have gotten the CRISPR treatment, too. And the gene editor has been used to reprogram human immune cells to fight cancer and to turn cancer cells against each other.
To snip or not to snip? That is the question
With CRISPR’s great power has come great controversy. That’s something Doudna and coauthor Samuel Sternberg warned in their 2017 book A Crack in Creation: The New Power to Control Evolution. Some think the gene editor might be used to help endangered species or to keep mosquitoes from carrying diseases. However, such efforts might even drive entire species extinct or create ecological disasters. Already scientists have wiped out small populations of mosquitoes in the lab.
Most controversially, a scientist in China edited genes in human embryos. The tests produced twin baby girls in 2018. Backlash against his actions was swift and vocal. Some people called for a ban on gene editing of babies.
CRISPR’s enormous power “means that we need to use it with great care,” says Claes Gustafsson. He chairs the Nobel Committee for Chemistry. “But it’s equally clear that this is a technology … that will provide humankind with great opportunities,” he said at an October 7 news conference.
Already, clinical trials are underway to test CRISPR/Cas9’s ability to treat cancer, sickle cell disease, inherited blindness and more. If successful, gene editing may treat, or even cure, previously untreatable genetic conditions.
CRISPR has even played a role in the coronavirus pandemic. Some diagnostic tests for COVID-19 and therapies in development are based on CRISPR tech.
Only five women other than Doudna and Charpentier have ever won the Nobel Prize in chemistry.
Says Doudna: “I think for many women there’s a feeling that their work will never be recognized as it might be if they were a man. I want to see that change, and I certainly hope this is a step in the right direction.”
Staff writer Maria Temming contributed to this story.







